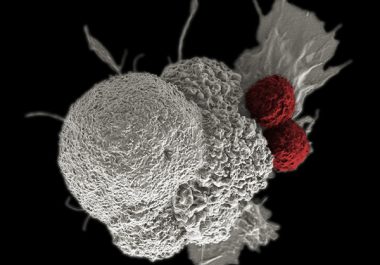
Immunotherapeutic Use Expanded to Include Head and Neck Cancer
The FDA expanded the use of an immunotherapy to include the treatment of certain patients with head and neck cancer.


The FDA expanded the use of an immunotherapy to include the treatment of certain patients with head and neck cancer.

The FDA approved a liquid biopsy – a test that uses a blood sample – to identify patients with metastatic non-small cell lung cancer.

Researchers urge better regulation to protect public health It’s no surprise that air pollution has been linked with lung cancer. A new study suggests that pollution is also associated with increased risk of mortality...
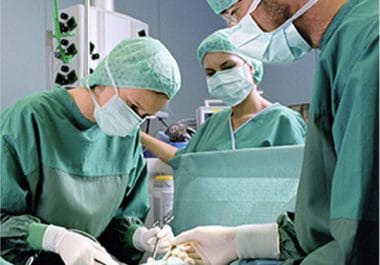
The amount of residual tumor left after surgery is an important prognostic factor for ovarian cancer patient survival. A newly devised tumor-specific fluorescent agent and imaging system guided surgeons in real time to remove...
Researchers examine the “oral microbiome” to look for clues to cancer risk. In a continuing effort to better understand how cancers develop, some researchers are looking into the mouth and finding that poor oral...

The types of fats that girls consume during adolescence could influence breast density, a risk factor for breast cancer, in young adulthood.

Maintaining an exercise routine, or even launching a new one, may improve outcomes for men diagnosed with prostate cancer.
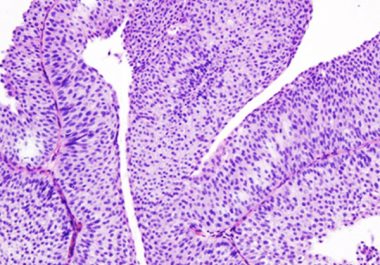
The May 2016 approval of atezolizumab provided the first new treatment in more than 30 years for patients with the most common type of bladder cancer.
The FDA expanded the use of an immunotherapeutic to include certain patients with Hodgkin lymphoma. Learn more about this approval.
The FDA added lenvatinib to the armamentarium for oncologists treating certain patients with renal cell carcinoma.