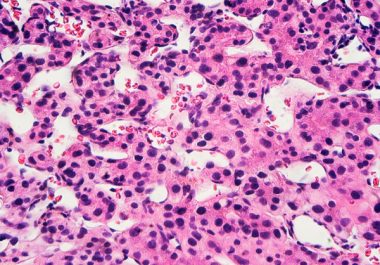
Aiming at Two Protein Targets to Treat Liver Cancer
The FDA approved a combination of an immunotherapy and a therapeutic that can stop tumors from growing blood vessels for certain patients with liver cancer.


The FDA approved a combination of an immunotherapy and a therapeutic that can stop tumors from growing blood vessels for certain patients with liver cancer.
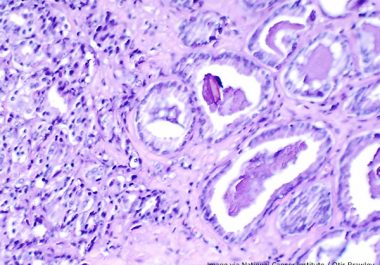
The FDA has expanded the use of the PARP-targeted therapeutic olaparib to include the treatment of certain patients with metastatic prostate cancer. The U.S. Food and Drug Administration (FDA) has expanded the use of...
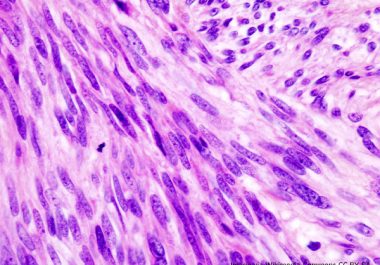
The FDA has approved a new molecularly targeted therapeutic for treating certain patients who have advanced gastrointestinal stromal tumors.

The FDA has expanded the use of a molecularly targeted therapeutic to include the treatment of certain patients with prostate cancer.
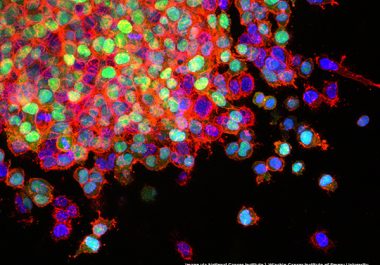
The FDA has approved a combination of the immunotherapeutics nivolumab and ipilimumab to treat certain patients with metastatic lung cancer.
The FDA has expanded the use of an immunomodulatory therapeutic to include the treatment of adults who have Kaposi sarcoma.
The FDA has approved an alternative dosing schedule for the immune checkpoint inhibitor pembrolizumab.
The FDA approved a new molecularly targeted therapeutic for certain patients with lung cancer driven by a mutation in the MET gene.
The FDA approved a type of molecularly targeted therapeutic called an antibody-drug conjugate for the treatment of metastatic triple-negative breast cancer.
The FDA has approved a new molecularly targeted therapeutic for treating certain patients with advanced or metastatic HER2-positive breast cancer.