Targeting Bile Duct Cancer
The first molecularly targeted therapeutic for use in the treatment of bile duct cancer has been approved by the FDA.


The first molecularly targeted therapeutic for use in the treatment of bile duct cancer has been approved by the FDA.
Treating a rare cancer with a chemotherapy gel.

The FDA has approved a molecularly targeted therapeutic for treating certain patients with neurofibromatosis type 1 who have inoperable tumors.
The FDA has approved a new combination of molecularly targeted therapeutics for treating metastatic colorectal cancer with a BRAF V600E gene mutation.
The FDA has approved a new combination of immunotherapy and chemotherapy as an initial treatment for extensive-stage small cell lung cancer.
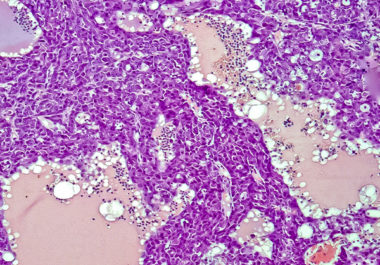
The FDA approved using a combination of nivolumab and ipilimumab, two immune checkpoint inhibitors, to treat certain patients with hepatocellular carcinoma.
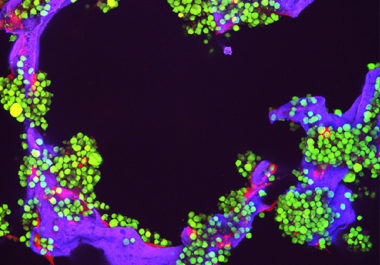
The FDA has approved a new therapeutic to treat adults with multiple myeloma whose cancer has progressed despite receiving at least two other treatments.
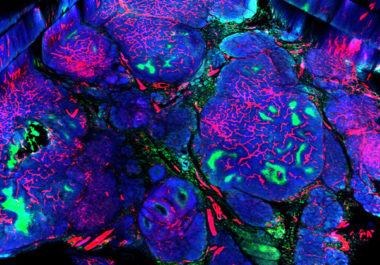
The FDA has expanded the use of the molecularly targeted therapeutic neratinib to include the treatment of certain patients with advanced or metastatic breast cancer
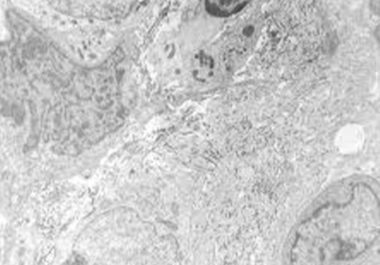
The FDA has approved a molecularly targeted therapy for epithelioid sarcoma, the first treatment approved for this rare form of soft tissue sarcoma.

The FDA has approved a molecularly targeted treatment for certain patients with gastrointestinal stromal tumors.