
January 7: The Week in Cancer News
Three out of four people with advanced colorectal cancer suffer significant financial hardship, and lung cancer patients who quit smoking experience increased survival.


Three out of four people with advanced colorectal cancer suffer significant financial hardship, and lung cancer patients who quit smoking experience increased survival.

The year 2021 defied our expectations in a variety of ways. The delta and omicron COVID-19 variants imposed unprecedented challenges on the health care system and threatened our hopes of an end to the...

When the COVID-19 pandemic forced much of the world to shut down, and many days seemed bleak, it was easy to joke about the allure of a splash of rum in a morning coffee,...

FDA approves drug to prevent graft versus host disease and decline in lung cancer deaths linked to screening.

The first classification of childhood tumors separating them from adult cancers will be published as a "blue book" by the World Health Organization.

When Abigail Johnston’s first child entered preschool six years ago, a vigilant pediatrician recommended an unusual strategy for minimizing germ exposure for the rest of the family. “Every day when my son came home,...

A new program specializes in concerns of gay and bisexual men with prostate cancer, and other cancer research news of the week.

All photos ©2021 AACR/Vera LaMarche/Whitney Thomas

Cancer is a very complex disease. Cancer cells often have the ability to rewire some of their functions to survive in harsh environmental conditions and bypass the toxic effect of therapies. But, paradoxically, some of the alterations...
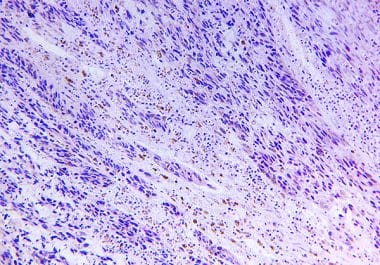
The FDA approved an immune checkpoint inhibitor for patients with stage IIB or IIC melanoma following surgical removal of their tumor. The U.S. Food and Drug Administration (FDA) approved the immunotherapeutic pembrolizumab (Keytruda) for...