
April 9: The Week in Cancer News
The launch of a campaign to address the decline in cancer screening rates, and more news of the week from Cancer Today.


The launch of a campaign to address the decline in cancer screening rates, and more news of the week from Cancer Today.

A study indicates that people are delaying medical care until they are eligible for Medicare, and more news of the week from Cancer Today.
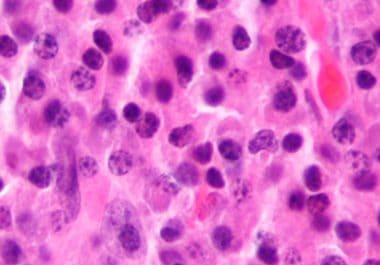
The FDA approved the first CAR T-cell therapy to treat certain adult patients with multiple myeloma. The U.S. Food and Drug Administration (FDA) has approved idecabtagene vicleucel (Abecma) to treat adult patients with relapsed...

Underrepresentation of members of minority groups in public genomic databases could lead to misleading test results, and more news of the week from Cancer Today.
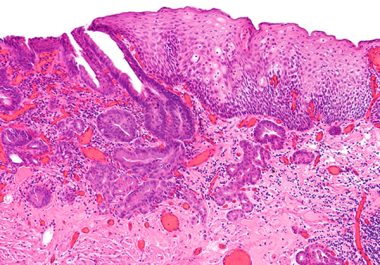
the immunotherapeutic pembrolizumab in combination with chemotherapy WAS APPROVED to treat certain adult patients with esophageal cancer. The U.S. Food and Drug Administration (FDA) recently approved the immune checkpoint inhibitor pembrolizumab (Keytruda) in combination...
A study indicates breast cancer centers often recommend earlier and more frequent screening than national guidelines, and more news of the week from Cancer Today.
With the August 2020 U.S. Food and Drug Administration approvals of the Guardant 360 CDx and FoundationOne Liquid CDx liquid biopsy tests to detect genetic abnormalities over multiple tumor types, the use of liquid...
Lung cancer screening recommendations expand to include more smokers, and more news of the week from Cancer Today.
By Nicholas Warren, PhD Lung cancer is the deadliest cancer in the United States, killing more than 135,000 Americans every year. More than half of all lung cancers are diagnosed after the cancer has...
The FDA approved the molecularly targeted therapeutic tivozanib to treat certain adult patients with the most common type of kidney cancer. The U.S. Food and Drug Administration (FDA) has approved tivozanib (Fotivda) to treat...