TIL Therapy Explained by Steven Rosenberg, MD, PhD
Much of the excitement revolving around cancer immunotherapy is focused on checkpoint inhibitors – drugs that “release the brakes” on T cells and enable them to attack tumors. Currently, the U.S. Food and Drug Administration (FDA) has approved seven checkpoint inhibitors for the treatment of various solid and hematologic cancers since 2011. While these treatments have led to unprecedented responses in some patients, many either do not respond or develop resistance to these drugs.
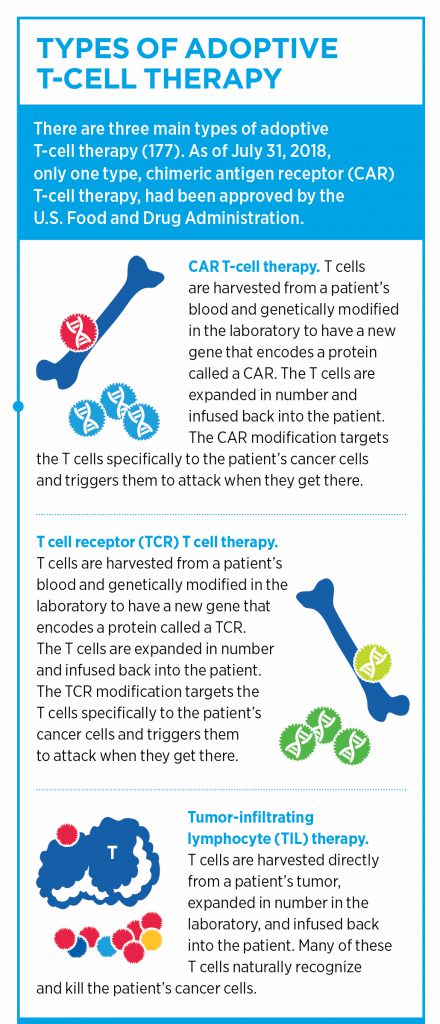
Adoptive cellular therapies (ACT) represent another class of immunotherapy, in which T cells are either isolated based on their tumor-specific antigen recognition or are genetically modified to target certain molecules present on cancer cells. These tumor-reactive T cells are then expanded and infused back into the patient.
Perhaps the most well-known type of ACT is chimeric antigen receptor (CAR) T-cell therapy. In this treatment, T cells are harvested from peripheral blood cells and are modified to express a receptor that can recognize a specific cell-surface protein on cancer cells. To date, the two FDA-approved CAR T-cell therapies, highlighted in this post, are engineered to target and destroy cells that express CD19 on their surface. Current CAR T-cell therapies are approved for the treatment of certain patients with non-Hodgkin lymphomas and leukemias.
Despite its many successes, CAR T-cell therapies haven’t proven effective in solid tumors so far. As hematologic malignancies make up only a small fraction of all cancers, research is necessary to adopt ACT to benefit patients with a wide variety of cancer types.
A type of ACT which predates CAR T-cell treatments is tumor-infiltrating lymphocyte (TIL) therapy. We spoke with immunotherapy pioneer Steven Rosenberg, MD, PhD, chief of surgery at the National Cancer Institute, about his work in the field and about his recent paper, published in Clinical Cancer Research.
How does TIL therapy work?
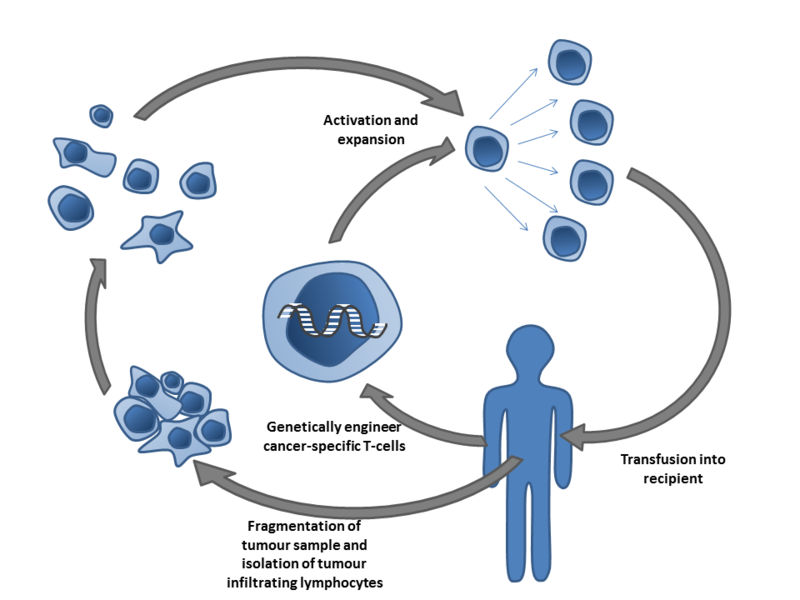
In ACT, tumor-specific T cells are isolated from unmodified T cells, as is seen in TIL therapy, or are genetically modified. These T cells are then expanded and infused back into the patient. Image by Simon Caulton via Wikimedia.
TILs are a collection of lymphocytes that have penetrated the stroma of a tumor. These TILs are largely comprised of T cells that are actively engaged in tumor destruction.
While other types of ACT utilize circulating T cells in the blood, TIL therapy relies on a tumor biopsy taken from the patient, Rosenberg explained. Following excision, DNA isolated from tumor biopsies are sequenced to identify mutations found in the cancer. The mutated neoepitopes are then inserted into autologous dendritic cells, a type of antigen-presenting cell, which are co-cultured with TILs isolated from the tumor biopsy. The TILs are then assayed for neoantigen-specific recognition through their secretion of cytokines, such as interferon gamma. TILs that can recognize the neoantigens are then selected, expanded, and transfused back into the patient. In short, TIL therapy utilizes T cells that already recognize and target a patient’s tumor as a treatment for their cancer, Rosenberg said.
“Intuitively, what better place to find T cells that are reacting against the cancer than in the stroma of the cancer itself?” said Rosenberg. “Back in 1988, we published our first work showing that TILs isolated from patients with metastatic melanoma could be expanded in the lab and returned to the patient, where they mediated cancer regression.”
Analysis of samples from melanoma patients treated with TIL therapy revealed that the T cells could recognize tumor neoantigens, Rosenberg explained. Furthermore, the majority of these neoantigens were unique to that individual cancer and not shared by other cancers, he noted. “It’s somewhat ironic that the very mutations that cause a certain cancer may turn out to be its Achilles’ heel and enable immunotherapy treatment,” said Rosenberg.
In addition to melanoma, TIL therapy has proven efficacious in patients with colorectal cancer, bile duct cancer, and breast cancer in preliminary studies.
Applying TIL therapy to ovarian cancer
In their recent paper, Rosenberg and colleagues evaluated tumor biopsies taken from seven women with metastatic ovarian cancer via whole exome and transcriptome sequencing. Once the mutations in the cancer were identified, they utilized an optimized high throughput method to determine which TILs could recognize the specific neoantigens in the tumor, Rosenberg explained. “There are no predictions involved in our method; we test every mutation found in the cancer to see if the patient’s T cells can recognize them. We also identify the T cell receptors (TCRs) that can perform the recognition of these cancer-specific mutations,” he said.
Of the seven patients, five had T cells that could recognize neoantigens found in their cancers, indicating that the majority of ovarian cancer patients in this study had tumor mutations that could be targeted by their own T cells.
“Over the years, we’re getting better and better at identifying the antigens that are recognized by the T cells,” explained Rosenberg. “We are currently developing methods that can identify additional types of mutations compared to the techniques that were utilized in this particular study.” As their methods become more sensitive, more tumor-reactive T cells will be identified, which will ultimately identify more patients that could be receptive to ACT, he added.
“The methods utilized in this study represent a blueprint for the isolation of T cells that can react against any type of cancer,” said Rosenberg. “Because all cancers have mutations, the techniques that we’ve described can potentially be used to identify T cells that react to mutations that occur in any tumor.”
TCR T-cell therapy
Another important finding of this study was that two patients had T-cell reactivity against TP53 “hotspot mutations,” or mutations to codons in the p53 gene that are commonly seen in cancers. Neoantigens from these common p53 mutations were presented via major histocompatibility complexes (MHCs) expressed by the same restriction element, or human leukocyte antigen (HLA) type. The identification of TCRs that can react to commonly mutated genes, such as p53, could lead to new therapeutics for patients that carry these mutations and express the appropriate HLA type, said Rosenberg.
“Potentially, if you identify a common p53 mutation in a patient that has the appropriate HLA type, you could use the TCRs that we’ve identified for treating multiple patients,” Rosenberg noted.
The identification of endogenous TCRs that recognize tumor-specific neoantigens is an essential step in another type of ACT, known as TCR T-cell therapy.
Unlike CAR T-cell therapy, which works by recognizing a specific antigen expressed on the surface of a cell through a novel receptor, TCR T-cell therapy borrows from endogenous TCRs that can recognize antigens presented via the MHC on tumors. While this method can be advantageous in that TCRs can recognize intracellular antigens, TCR T-cell therapy is dependent on a specific HLA for antigen recognition, which limits its utility, as not all individuals express the same HLA types, Rosenberg noted.
Because TCR T-cell therapy utilizes peripheral lymphocytes, this represents an advantage over TIL therapy, he said. “Peripheral lymphocytes can be a lot more active and have a higher proliferative potential compared to TILs that have been repetitively stimulated in a patient and have lost some of their ability to divide. If we could put these TCR genes into peripheral lymphocytes, many of whom are naïve, these cells can have an explosive proliferative potential and could offer a more effective treatment,” he explained. Indeed, there is an ongoing clinical trial that is evaluating this method in patients with advanced solid tumors.
“The opportunities to improve cancer immunotherapy treatments are very exciting. We are able to utilize a whole new tool – a patient’s own T cells – to explore the body’s reactions to cancer, and I think this is one of the most exciting areas of modern research in the cancer arena,” Rosenberg said.