MASTL: A Chromosomal Stability Gene and Exploitable Vulnerability in Prostate Cancer
Metastatic prostate cancer (PCa) remains a leading cause of death in men worldwide (1). Although effective treatments exist, therapy resistance in PCa is common. Dr. Rodriguez-Bravo and her research team recently uncovered high chromosomal instability (CIN) and increased mitotic kinase levels in castration and chemotherapy resistant metastatic PCa. They uncovered that upregulation of mitotic kinase MASTL, driven by transcriptional rewiring mechanisms, was a survival adaptation and therapeutic vulnerability in metastatic PCa.
Veronica Rodriguez-Bravo, PhD, is an associate professor in the Biochemistry and Molecular Biology, and Urology departments at Mayo Clinic, Rochester, Minnesota. She received the Friends of the AACR Foundation Early Career Investigator Award in 2018 explaining, “The support provided by AACR was crucial to jump deep into an area not explored mechanistically before in prostate cancer. It allowed us to think outside of the box and follow an unconventional avenue to answer a strong biological question: How do metastatic tumors carrying high chromosomal aberration content manage to continue thriving, growing, and resisting therapies?”
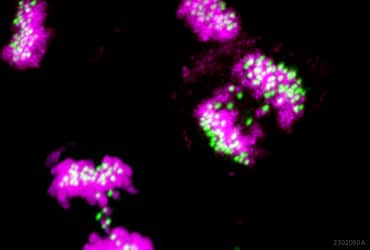
In the study, recently published in Cell Reports Medicine, Dr. Rodriguez-Bravo and her colleagues first assessed and identified high CIN in metastatic PCa by measuring chromosomal mis-segregation in primary and metastatic tissue samples as well as the fraction of genome altered in primary and metastatic patient datasets (2). As chromosomal mis-segregation errors originate during mitosis, the researchers then sought to determine the status of mitotic chromosomal stability maintenance pathways and identified a group of mitotic kinases upregulated in metastatic PCa. Through further interrogation in cell line models representing the high CIN and mitotic kinase gene expression observed in metastatic PCa, the researchers were able to identify top chromosomal stability genes (including MASTL) to which metastatic chemotherapy resistant PCa cells became selectively dependent to survive.
By performing quantitative phosphoproteomic analysis, the research team then compared the phosphorylation changes during cell division between parental, and chemotherapy-resistant PCa cell lines. Cell lines were differentially labeled to perform stable isotope labelling by amino acids in culture (SILAC) analysis, and arrested in mitosis, before phosphopeptide enrichment, mass spectrometry and bioinformatic analysis to determine statistically significant differences between the cells. Notably, this approach also identified increased MASTL activity in chemotherapy resistant PCa cells.
Dr. Rodriguez-Bravo and her colleagues hypothesized that transcriptional upregulation of MASTL was a CIN adaptation mechanism in metastatic therapy refractory PCa, which could be exploited therapeutically. They showed that CIN was elevated above a tolerable level when MASTL was inhibited with the first generation MASTL inhibitor, GKI-1. The researchers also found that MASTL inhibition was more cytotoxic in the metastatic CIN high cell lines than in parental PCa cell lines. These findings were also validated in vivo, using cell line and patient-derived xenograft pre-clinical models generated from circulating tumor cells of metastatic PCa patients previously treated with anti-androgen and/or taxane.
Explaining the importance of the study, Dr. Rodriguez-Bravo said, “We believe our study provides biological and mechanistic evidence that chromosomal instability adaptation is an acquired vulnerability of aggressive prostate tumors, and possibly other tumors too, that can be therapeutically exploited. The consequences of chromosomal instability are context dependent; at low levels it can provide adaptive advantages to tumors, but we and others propose that high levels of it can be detrimental for tumors, unless they adapt somehow, as we show in this study.”
She went on to say, “The funding I received from the AACR allowed us to take risks and explore unusual questions in the field of chromosomal instability and lethal prostate cancer. We are very happy to be able to contribute to the mission to understand cancer better, and to find new routes for therapeutics and improve the lives of patients.”
More current findings on the basic biology of prostate cancer and how these may be translated to the clinic will be covered in the upcoming AACR Special Conference: Advances in Prostate Cancer Research meeting. Taking place March 15-18, 2023, in Denver, Colorado, and available online, the meeting will provide a unique opportunity to interact with basic scientists, translational researchers and clinicians in the field.
References:
- Siegel RL, Miller KD, Fuchs HE, and Jemal A. Cancer statistics, 2022. CA. Cancer J. Clin 2022; 72: 7–33. doi: 10.3322/caac.21708
- Soni RK, Li Z, Hendrickson RC, Schiewer MJ, Kelly WK, Sternberg CN, Luo J, et al. Harnessing transcriptionally driven chromosomal instability adaptation to target therapy-refractory lethal prostate cancer. Cell Reports Medicine 2023; 4: 100937. doi: 10.1016/j.xcrm.2023.100937