Impact of the AACR-BCRF Partnership
The AACR and Breast Cancer Research Foundation (BCRF) have been collaborating since 2007 to support the most promising and innovative breast cancer-focused research. This partnership has yielded significant outcomes, as indicated below.
Potential therapeutic targets for treatment and interception
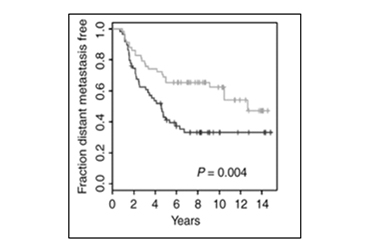
Ref: Clin Cancer Res 17: 2874-2884. Kaplan-Meier plots of distant metastasis events in luminal B breast cancer patients (black, high eIF4E mRNA; gray, low eIF4E mRNA)
2009 BCRF-AACR Grant recipient Wilson Miller Jr. showed that high expression of eukaryotic initiation factor 4E (eIF4E) mRNA was correlated with poor prognosis in luminal B breast cancer. He and his colleagues subsequently reported that genetic and pharmacologic inhibition of eIF4E reduced breast cancer cell migration, invasion and metastasis.
Currently, eIF4E inhibitors for breast cancer are actively being investigated.
2014 BCRF-AACR Grant recipient Fariba Behbod demonstrated the role of Bcl-9 in promoting invasive progression of ductal carcinoma in situ (DCIS). She and her group subsequently elucidated that Bcl-9 complexed with Stat3 regulated enhancers whose targets are involved in cellular growth, invasion, and migration and that carnosic acid, a natural compound in rosemary extract, previously shown to inhibit Bcl-9/β-catenin-dependent transcription, significantly inhibited DCIS progression in vivo.
Novel insights into breast cancer metastasis
2012 BCRF-AACR Grant recipient Shizhen Emily Wang hypothesized that certain blood-borne microRNAs are associated with breast cancer metastasis.
She and her group had previously observed that higher levels of circulating miR-122 in early-stage breast cancer were predictive of metastatic progression. With the support of her BCRF-AACR grant, they showed that breast cancer cells secrete miR-122. The secreted miR-122 reprograms glucose consumption in premetastatic niche tissues. Inhibition of the microRNA decreased the incidence of metastasis in vivo. Two miR-122 inhibitors have been tested in Phase Ib or Phase II clinical trials; no significant adverse effects or long-term safety issues were observed. In addition to miR-122, Dr. Wang also found that metastatic breast cancer cells (and not primary tumors) secreted miR-105. This secreted miR-105 downregulated the expression of tight junction proteins in endothelial cells, consequently disrupting blood vessel integrity and promoting metastasis.
Pregnancy and breast cancer
Women who have experienced at least one full-term pregnancy before the age of 30 appear to have a lower risk of developing breast cancer. 2018 BCRF-AACR Grant Recipient Camila dos Santos confirmed previous findings that parous mammary glands in vivo are resistant to premalignant lesion development, even in the midst of overexpression of the oncogene cMyc. Instead, post-pregnancy mammary epithelial cells assumed a senescence-like state in response to cMyc overexpression.
Results of her epigenome characterization studies suggest that pre- and post-pregnancy mammary epithelial cells (MECs) regulate distinct molecular pathways during mammary tissue homeostasis. In addition, the H3K27ac landscape changes that were detected in luminal MECs during pregnancy were stably maintained in subsequent pregnancies.
BCRF-AACR grant recipients also have contributed to the understanding of pregnancy-associated breast cancer (PABC). PABC has been traditionally defined as breast cancer diagnosed during pregnancy or within one year of delivery.
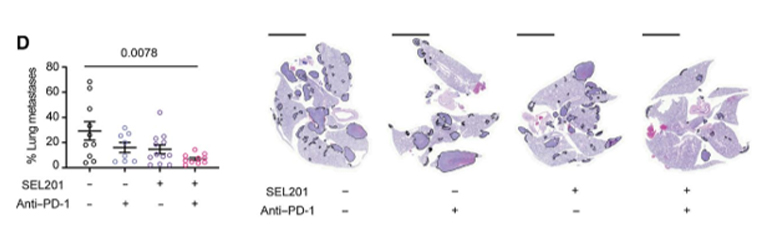
2009 BCRF-AACR Grant Recipient Virginia Borges has led the discussion in expanding the definition of PABC to include women who were diagnosed up to 10 years after childbirth. In a cohort study of 701 women 45 years or younger with stage I-III breast cancer, patients who had breast cancer up to 10 years after childbirth had more than a 2-fold increase risk of metastasis compared with nulliparous patients. Recent work by a BCRF-AACR grant recipient Wilson Miller Jr (mentioned above) showed that the increased propensity for metastasis of PABC cells can be explained, at least in part, by activation of the MNK1/2-eIF4E signaling pathway. He and his group showed that combining anti-PD-1 antibody with the MNK1/2 inhibitor SEL201 significantly inhibited metastasis in a PABC mouse model – an observation that was not observed with the single agents.
Better tumor models
2007 BCRF-AACR Grant Recipient Alana Welm had previously identified that macrophage-stimulating protein (MSP) promotes breast cancer metastasis in vivo, especially to the bones. Supported by her grant, she sought to determine if MSP inhibitors have therapeutic potential.
In the course of her work, she and her group developed the methodology to transplant fresh breast cancer patient tumor samples into mammary fat pads of NOD/SCID mice. In contrast to patient-derived xenograft models, majority of tumor grafts metastasized in a pattern that was similar to what was observed in the original patients.