Editors’ Picks, October 2025: Drug Delivery Technology, Anticancer Effects of Alzheimer’s Protein, and More
Is there anything so scary as missing out on the latest in cancer research? We at Cancer Research Catalyst certainly don’t think so. Braving the ghostly chill in the air this all hallows’ eve, we’re bringing you October’s round of editors’ picks from the journals of the American Association for Cancer Research (AACR)—what a treat!
As for the tricks these researchers have up their sleeves, why, there’s a whole bag of goodies. A clinical trial showed that a new small molecule drug sensitized STK11-mutant non-small cell lung cancer to immune checkpoint inhibition, and on the therapeutic front, interesting developments continue to be made. In one study, researchers developed a bispecific antibody to target basal cell prostate cancer, and in another, researchers published their work not on antibody-drug conjugates but small-molecule-drug conjugates, which fuse a drug payload with an engineered ligand to seek out tumor-associated receptors.
And if you’ve heard about the apparent inverse relationship between Alzheimer’s disease and cancer, one of this month’s picks provided insights into this relationship’s mechanistic basis. Researchers have uncovered the mechanisms of how the Alzheimer’s-related amyloid-β precursor protein may serve an anticancer function by improving T cells’ antitumor performance.
All the abstracts are printed in full below, and, as always, these selections from AACR journals are available in full and for free at the provided links for a limited time.
Journal: Blood Cancer Discovery
Multiple myeloma remains incurable despite advances in immunotherapies like chimeric antigen receptor (CAR) T-cell therapy. This study investigates the role of metabolites and gut microbiota in clinical outcomes in patients treated with the humanized B-cell maturation antigen (BCMA)-directed CAR-T therapy ARI0002h. Stool metabolites, particularly succinate, were associated with CAR T-cell phenotypes and persistence in patients. In CAR T-cell culture, succinate supplementation enhanced CD4+ central memory phenotype and respiratory capacity. In a murine myeloma model, a succinate-enhancing diet significantly improved CAR T-cell persistence and showed a trend toward better tumor control. Furthermore, Acidaminococcaceae, Monoglobaceae, or Akkermansiaceae, along with specific metabolites, were associated with CAR T-cell clinical outcomes. These multimodal profiles were integrated into response models, including one that identified patients likely to achieve a complete response by days 100 and 180 after infusion. These findings suggest that metabolites and gut microbiota correlate with CAR T-cell therapy responses and can be a valuable tool for risk assessment.
Significance: This study integrates microbial profiles into response models, providing a tool to identify patients with multiple myeloma who may benefit from BCMA-directed CAR T-cell therapy optimization by identifying bacterial taxa and metabolites associated with CAR T-cell persistence and therapeutic outcomes.
This article was highlighted in the September issue.
Journal: Cancer Discovery
AAnet Resolves a Continuum of Spatially Localized Cell States to Unveil Intratumoral Heterogeneity
Identifying functionally important cell states and structure within heterogeneous tumors remains a significant biological and computational challenge. Current clustering- or trajectory-based models are ill-equipped to address the notion that cancer cells reside along a phenotypic continuum. We present Archetypal Analysis network (AAnet), a neural network that learns archetypal states within a phenotypic continuum in single-cell data. Unlike traditional archetypal analysis, AAnet learns archetypes (AT) in a simplex-shaped neural network latent space. Using preclinical and clinical models of breast cancer, AAnet resolves distinct cell states and processes, including cell proliferation, hypoxia, metabolism, and immune interactions. Primary tumor ATs are recapitulated in matched liver, lung, and lymph node metastases. Spatial transcriptomics reveals archetypal organization within the tumor and intra-archetypal mirroring between cancer and adjacent stromal cells. AAnet identifies GLUT3 within the hypoxic AT that proves critical for tumor growth and metastasis. AAnet is a powerful tool, capturing complex, functional cell states from multimodal data.
Significance: Defining critical cell states among cells that reside along a phenotypic continuum is a current biological and computational challenge. In this study, we present AAnet, a neural network that learns archetypal cell states of cancer cells. AAnet defines discrete spatially localized ATs that resolve intratumoral heterogeneity.
This article was highlighted in the October issue.
Journal: Cancer Epidemiology, Biomarkers and Prevention
Background: Cancer incidence data collected by cancer registries in the United States and Canada are submitted to the North American Association of Central Cancer Registries, which publishes annual case counts for the two countries. To allow time to collect and report cases, counts for a given diagnosis year are initially published two years after the end of that year and updated annually. Initial counts typically underreport cases compared with updated counts due to reporting delays, potentially biasing estimated incidence trends.
Methods: Existing methods for estimating “delay-adjusted” counts are modified for this heterogeneous group of registries exhibiting different patterns of reporting delay. The new method can be applied to individual registries and combined to produce delay-adjusted rates for the entire population, as well as for geographic or demographic subpopulations.
Results: Steps involved in estimating delay-adjusted counts are illustrated for liver and intrahepatic bile duct cancer in White males, in which delay-adjusted rates exhibit a stabilized trend, in contrast to the rapid decline seen in observed (unadjusted) rates. Additionally, the new delay model reveals reporting delays varying across cancer sites, race, and ethnicity. Finally, an extended model provides validated delay-adjusted rates from preliminary data that reduces reporting time from 2 years to 1 year.
Conclusions: Adjusting for reporting delay provides more accurate estimates of cancer incidence trends. The proposed method addresses practical issues of model implementation and continues the evolution of delay adjustment in cancer registries.
Impact: The new model extends the use of delay adjustment to an important source of cancer surveillance statistics.
This article was highlighted in the October issue.
Journal: Cancer Immunology Research
Macrophages expressing Trem2 play a pivotal role in promoting nonalcoholic steatohepatitis (NASH; also known as metabolic dysfunction–associated steatohepatitis) progression to hepatocellular carcinoma (HCC). Despite the widespread clinical use of anti–PD-1 immune checkpoint blockade, its therapeutic efficacy in NASH-driven HCC remains suboptimal. This study investigates the mechanisms by which NAM Trem2 influences the response of NASH-driven HCC to immunotherapy. Clinical analysis revealed that elevated Trem2 expression in NASH is positively correlated with the accumulation of neutrophil extracellular traps (NET) and infiltration of PD-1+Eomes+CD8+ T cells and regulatory T cells. Myeloid-specific knockout of Trem2 (Trem2Δmye) led to impaired macrophage reprogramming, resulting in the accumulation of proinflammatory Ly6ChiCX3CR1lo macrophages, which enhanced degradation of NETs in NASH. Trem2Δmye also disrupted TGFβ production via P-Syk–dependent efferocytosis, collectively suppressing the differentiation of PD-1+Eomes+CD8+ T cells and regulatory T cells. The efficacy of anti–PD-1 therapy in inhibiting NASH-driven HCC progression was also significantly enhanced by Trem2Δmye, primarily through the downregulation of Treg CXCR4 expression mediated by increased NET degradation. These therapeutic effects were further amplified when combined with the CXCR2 inhibitor AZD5069. Our findings identify Trem2 as a central regulator of the NASH-driven HCC immunosuppressive niche and suggest a combinatorial therapeutic strategy that targets both myeloid reprogramming and NETosis to overcome immunotherapy resistance in metabolic liver cancer progression.
This article was featured on the cover of the October issue.
Journal: Cancer Prevention Research
Data on the care of pediatric patients with hereditary polyposis syndromes (HPS) including familial adenomatous polyposis (FAP), juvenile polyposis syndrome, and Peutz–Jeghers syndrome are limited. We aim to describe the current practice patterns for HPS. An anonymous survey was distributed to pediatric gastroenterologists, pediatric surgeons, and adult colorectal surgeons. A total of 150 pediatric gastroenterologists and 129 surgeons started the survey, and 80 gastroenterologists and 70 surgeons completed the survey. A total of 62% of pediatric gastroenterologists identified that their clinical care most closely follows the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition position statement and 42% of surgeons reported following the National Comprehensive Cancer Network guidelines (P < 0.001). For gastroenterologists, 76% currently manage FAP (61% follow 1–5 patients) and 34% recommended genetic testing at birth or first presentation. At 10 to 14 years, 91% recommended initial colonoscopy. High-grade dysplasia (78%) was the most important factor for surgical referral for colectomy. A total of 43% reported documenting the number of rectal polyps and 31% referred to a surgeon for <50 polyps. Seventy-five percent manage juvenile polyposis syndrome and 56% manage Peutz–Jeghers syndrome. For surgeons, 81% currently manage FAP (56% follow 1–5 patients) and 68% follow patients <18 years. Twelve to 15 years was the most common age (47%) at colectomy. High-grade dysplasia (57%) was the most important factor for surgery. In the previous 12 months, 56% had not performed a colectomy. Ileal pouch–anal anastomosis was the most common reported surgery for FAP. Pediatric gastroenterologists and surgeons typically manage few pediatric patients with HPS, with significant heterogeneity and deviation from guidelines. Continued medical education is critical to standardizing care for pediatric HPS.
Prevention Relevance: Appropriate screening and surveillance in pediatric hereditary polyposis are critical in the early detection of intestinal cancers.
A related commentary was published, and the article was featured on the cover of the October issue.
Journal: Cancer Research (October 1 Issue)
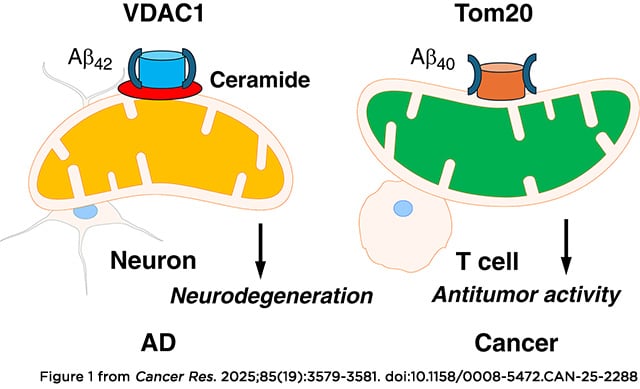
Patients with Alzheimer’s disease (AD) have a decreased incidence of cancer, with a cross-sectional analysis of a nationwide sample of adults finding 21-fold higher odds of cancer diagnosis in non-AD individuals compared with those with AD. In this study, we demonstrated that mitochondrial localization of AD-associated amyloid-β precursor protein (APP) and its cleavage product amyloid-β 40, but not mutant APP that lacks a mitochondrial localization signal, inhibits lipid stress–mediated hyperactive mitophagy in aging T cells, improving their antitumor functions. Growth of melanoma xenograft or carcinogen-induced oral cancer models was highly reduced in AD mice. Additionally, adoptive cell transfer–based immunotherapy using aging T cells isolated from AD mice suppressed tumor growth. The metabolic signature of stress-dependent mitophagy in T cells showed fumarate depletion, which was linked to decreased succination of Parkin and enhanced mitochondrial damage. Mechanistically, APP interaction with the TOMM complex at the outer mitochondrial membrane attenuated trafficking of ceramide synthase CerS6 to mitochondria in aging AD T cells, preventing ceramide-dependent mitophagy. Thus, APP restored mitochondrial fumarate metabolism and Parkin succination, improving antitumor functions of AD T cells in vitro and in vivo. Exogenous fumarate supplementation or healthy AD mitochondria transfer functionally mimicked the AD/APP phenotype in aging T cells, enhancing their antitumor activity to control tumor growth. Moreover, T cells isolated from aging donors showed elevated mitophagy with fumarate depletion, which was restored in T cells isolated from age-matched patients with AD. Together, these findings show that AD protects T cells against ceramide-dependent mitophagy and fumarate depletion to enhance antitumor functions.
Significance: The reduced cancer risk in Alzheimer’s disease patients is mediated by the amyloid-β 40 peptide, which inhibits aging-dependent mitophagy in T cells to improve antitumor immunity.
A related commentary was published in the October 1 issue.
Journal: Cancer Research (October 15 Issue)
Patients with non–small cell lung cancer (NSCLC) with loss of the tumor suppressor gene STK11 are resistant to immune checkpoint therapies like anti–PD-1. In this study, we conducted an in vivo CRISPR screen that identified histone deacetylase 1 as a target to reverse anti–PD-1 resistance driven by loss of STK11 and developed TNG260, a potent small-molecule inhibitor of the CoREST complex with selectivity exceeding previously generated inhibitors in this class in preclinical studies. Treatment with TNG260 led to increased expression of immunomodulatory genes in STK11-deficient cancer cells. When combined with anti–PD-1, TNG260 induced immune-mediated stasis and/or regression in STK11-deficient syngeneic tumor models and autochthonous NSCLC models. In the tumors of patients with STK11-deficient cancers in a clinical trial (NCT05887492), treatment with a combination of TNG260 and pembrolizumab increased intratumoral histone acetylation, PD-L1 tumor proportion scores, and T-cell infiltration into the tumor microenvironment. This study illustrates a promising treatment strategy for addressing immune evasion in patients with STK11-mutant NSCLC.
Significance: Targeting CoREST with TNG260 sensitizes STK11-deficient non-small cell lung cancer to anti-PD-1 immunotherapy, offering a potential treatment for patients not served by existing therapies.
A related commentary was published in the October 15 issue.
Journal: Clinical Cancer Research (October 1 Issue)
Purpose: Nelitolimod (previously SD-101) is a toll-like receptor 9 agonist. We assessed whether intratumoral nelitolimod plus pembrolizumab potentiates antitumor activity in patients with advanced melanoma who had not previously received anti–PD-1/PD-L1 therapy.
Patients and Methods: Patients with advanced melanoma who were naïve to anti–PD-1/PD-L1 therapy received either nelitolimod 2 mg injected into 1 to 4 lesions or nelitolimod 8 mg injected weekly into a single lesion for 4 weekly doses and then every 3 weeks. Pembrolizumab 200 mg was administered intravenously every 3 weeks.
Results: Forty-five patients received nelitolimod 2 mg and 41 patients received nelitolimod 8 mg per injection. The objective response rate (ORR) was 76% in the 2-mg group and 49% in the 8-mg group. In those with distant metastases, ORRs in both treatment groups were similar to the overall ORRs. In the 2-mg group, treatment responses were similar in those with PD-L1–positive tumors and those with PD-L1–negative tumors. The progression-free survival rate at 18 months (landmark) was 62% in the 2-mg group and 40% in the 8-mg group. Forty-four patients (100%) in the 2-mg group and 37 patients (95%) in the 8-mg group experienced a treatment-related adverse event with either drug; overall, 31 patients (37%) had a grade 3 or 4 treatment-related adverse event related to either study drug.
Conclusions: In patients with anti–PD-1/PD-L1 treatment-naïve advanced melanoma, nelitolimod plus pembrolizumab induced objective responses, including in PD-L1–negative tumors. The treatment combination warrants further study in advanced melanoma.
Journal: Clinical Cancer Research (October 15 Issue)
Purpose: Mouse double minute 2 (MDM2) is an E3 ubiquitin ligase that degrades the tumor suppressor p53. In cancers, MDM2 amplification (MDM2amp) leads to overexpression of MDM2, inducing p53 degradation and a p53-null phenotype even in the absence of TP53 mutations. We report here the preclinical and clinical activities of milademetan, a potent and selective oral small-molecule inhibitor of the MDM2–p53 interaction, in MDM2amp, TP53 wild-type (WT) solid tumors.
Patients and Methods: Milademetan was tested against a variety of cell line and xenograft tumor models. This supported a phase II basket study (MANTRA-2) in patients with advanced MDM2amp, TP53-WT solid tumors. The primary endpoint was the objective response rate, and key secondary endpoints included progression-free survival and adverse events.
Results: Milademetan showed potent activity against MDM2amp, TP53-WT laboratory models. In the phase II trial, 40 patients received milademetan, 31 of whom had centrally confirmed molecular testing. The best overall response was 19.4% (6/31) with one confirmed response (3.2%) and five unconfirmed partial responses, including a patient with endometrial stromal sarcoma who achieved a 100% target lesion reduction. The median progression-free survival was 3.5 months (95% confidence interval, 1.8–3.7). Grade 3 or 4 adverse events observed included thrombocytopenia, neutropenia, anemia, leukopenia, and diarrhea.
Conclusion: Milademetan had a manageable safety profile and achieved responses against a variety of refractory MDM2amp, TP53-WT solid tumors, but tumor reductions were short-lived. Subsequent efforts should focus on combination strategies, further biomarker refinement, or novel MDM2 targeting approaches to achieve more durable clinical benefit.
Journal: Molecular Cancer Research
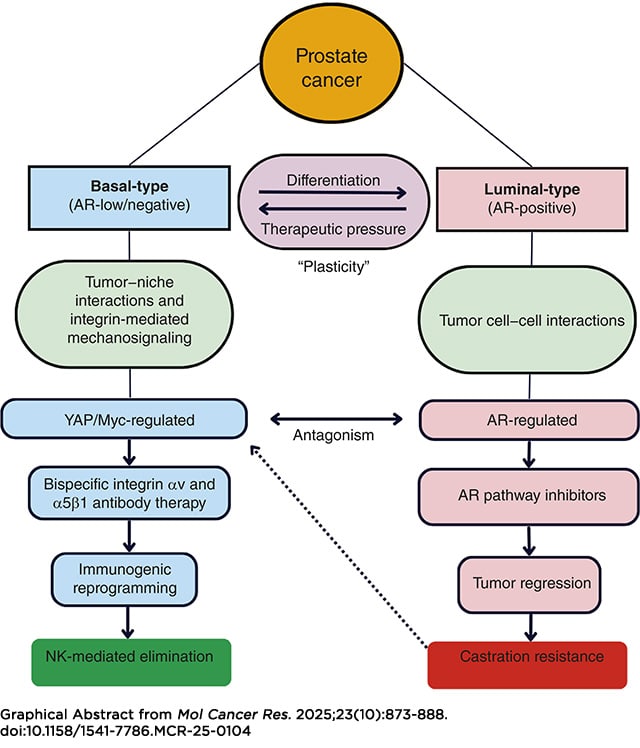
Integrin α5β1 and αv cross-talk in chemotaxis, and clonogenic survival of prostate cancer cells is abrogated by a bispecific α5β1/αv antibody (BsAbα5β1/αv), which uniquely induces internalization and lysosomal degradation of target integrins. We hypothesized that the BsAbα5β1/αv inactivates pathologic mechanosignaling pathways that correlate with integrin expression from patient samples. Mechanistic studies indicate that the BsAbα5β1/αv uniquely reverses Yes-associated protein, β-catenin, and focal adhesion kinase nuclear localization compared with monospecific integrin α5β1 and αv antibody controls in basal-type androgen receptor–negative prostate cancer cells. Dual integrin αv and α5 knockdown alone phenocopied the BsAbα5β1/αv effect. Following BsAbα5β1/αv treatment, Assay for Transposase-Accessible Chromatin using sequencing studies indicated the chromatin accessibility to TEAD and AP-1 family members was markedly reduced. In vitro and in vivo RNA sequencing indicated downregulation of Myc/E2F, TGF-β, and epithelial–mesenchymal transition and upregulation of type I and II IFN transcriptomic pathways. The BsAbα5β1/αv induced CXCL10 and CCL5 cytokine secretion, immune-infiltration of tumors, and NK cell–mediated elimination of the basal-type prostate cancer xenografts in nude mice. αv integrin was highly expressed and principally correlated with the Myc signaling pathway in rapid autopsy tissue microarrays, consistent with correlative data from the SU2C metastatic castration-resistant prostate cancer and Deutsches Krebsforschungszentrum early-onset prostate cancer cohorts. These studies connect integrin signaling with the central biology of basal-type and castration-resistant prostate cancers and define a novel therapeutic strategy that controls critical immunosuppressive pathways.
Implications: Dual integrin α5β1/αv targeting with a bispecific antibody represents a novel therapeutic strategy that reprograms the epigenetic and transcriptomic signatures of basal-type prostate cancer with induction of immunologic tumor control.
This article was highlighted in the October issue.
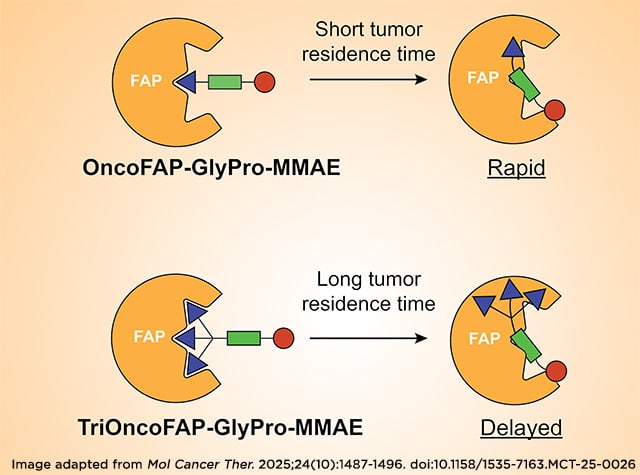
Journal: Molecular Cancer Therapeutics
Antibody–drug conjugates are one of the most diffused targeted therapeutic modalities for cancer treatment and consist of a tumor-targeted monoclonal antibody connected to a cytotoxic payload, which is released selectively at the tumor site. Small molecule–drug conjugates (SMDC) represent an alternative approach, in which the antibody is replaced by a tumor-homing small organic ligand. Thanks to their small molecular size, SMDCs are characterized by rapid extravasation and enhanced penetration in solid tumors compared with antibody–drug conjugates. We recently developed SMDCs targeting fibroblast activation protein (FAP), a cell surface endopeptidase abundant in the tumor microenvironment, using the highly specific FAP inhibitor OncoFAP as a targeting moiety. In this study, we compared the tumor-targeting properties and in vivo activity of SMDCs based on OncoFAP against products based on a stronger FAP inhibitor (i.e., trivalent OncoFAP), aiming to tune the release kinetic of the cytotoxic payload to the neoplastic site. We compared the kinetic profiles of the monovalent and trivalent derivatives of OncoFAP through in vivo and ex vivo biodistribution and therapy studies. The distinct in vivo monomethyl auristatin E (MMAE) release obtained for OncoFAP–GlyPro–MMAE and TriOncoFAP–GlyPro–MMAE did not lead to substantial differences in therapeutic efficacy in a preclinical FAP-positive cancer model.
This article was highlighted in the October issue and was also featured on the cover.
Journal: Cancer Research Communications
GPSai: A Clinically Validated AI Tool for Tissue of Origin Prediction during Routine Tumor Profiling
A subset of cancers present with unclear or potentially incorrect primary histopathologic diagnoses, including cancers of unknown primary (CUP). We aimed to develop and validate an artificial intelligence (AI) tool, Genomic Probability Score AI (GPSai™), which predicts tumor tissue of origin in CUP and flags potential misdiagnoses for additional workup during routine molecular testing. The GPSai model was trained on whole exome and whole transcriptome data from 201,612 cases submitted for tumor profiling at Caris Life Sciences. Retrospective (N = 21,549) and prospective (N = 76,271) validations were performed. The clinical impact was evaluated over 8 months of live testing and through physician surveys. GPSai demonstrated 95.0% accuracy in non-CUP cases and reported on tumor tissue of origin in 84.0% of CUP and 96.3% of non-CUP cases. During the initial 8 months of implementation, GPSai changed the diagnosis on 704 patients (0.88% of all profiled cases), which were supported by orthogonal evidence including imaging, IHC, mutational signatures, hallmark fusions, or viral reads. Diagnosis changes prompted changes in targeted therapy eligibility based on level 1 clinical evidence in 86.1% of cases (n = 606/704). A majority (89.7%; n = 87/97) of physician responses indicated acceptance of the GPSai results, and 53.6% (n = 52/97) of responses stated that the results prompted a change in treatment plan. GPSai accurately identifies tumor tissue of origin and has the potential for clinical impact in a small but meaningful subset of patients with CUP or pathologically ambiguous tumors. Our results support the integration of this AI tool into routine molecular testing to improve diagnostic accuracy and guide subsequent therapeutic decisions.
Significance: Our findings show that GPSai, a deep learning–based tool, can support the identification of primary tumor sites with high accuracy in conjunction with orthogonal evidence. Its integration into routine tumor profiling furthermore allows simultaneous biomarker identification. Analysis of real-world implementation of GPSai shows that it enhances diagnostic accuracy, including resolution of CUP cases, and prompts clinically relevant therapeutic recommendation changes without requiring additional specimen.