Editors’ Picks: July Highlights From AACR Journals
Grab a cold drink and escape the midsummer heat with July’s rendition of Editors’ Picks.
This month, the editors highlighted the role of alternative splicing in pancreatic cancer, the association between physical activity and cancer, and multiple combination therapies of investigational drugs.
The abstracts of the featured articles are included below. For complete details, follow the links to the full-text articles, which are freely available for a limited time.
Journal: Blood Cancer Discovery
The MYC oncoprotein is activated in a broad spectrum of human malignancies and transcriptionally reprograms the genome to drive cancer cell growth. Given this, it is unclear if targeting a single effector of MYC will have therapeutic benefit. MYC activates the polyamine–hypusine circuit, which posttranslationally modifies the eukaryotic translation factor eIF5A. The roles of this circuit in cancer are unclear. Here we report essential intrinsic roles for hypusinated eIF5A in the development and maintenance of MYC-driven lymphoma, where the loss of eIF5A hypusination abolishes malignant transformation of MYC-overexpressing B cells. Mechanistically, integrating RNA sequencing, ribosome sequencing, and proteomic analyses revealed that efficient translation of select targets is dependent upon eIF5A hypusination, including regulators of G1–S phase cell-cycle progression and DNA replication. This circuit thus controls MYC’s proliferative response, and it is also activated across multiple malignancies. These findings suggest the hypusine circuit as a therapeutic target for several human tumor types.
This article is highlighted in the July issue. A related commentary can be found here.
Journal: Cancer Discovery
Splicing Factor SRSF1 Promotes Pancreatitis and KRASG12D-Mediated Pancreatic Cancer

Inflammation is strongly associated with pancreatic ductal adenocarcinoma (PDAC), a highly lethal malignancy. Dysregulated RNA splicing factors have been widely reported in tumorigenesis, but their involvement in pancreatitis and PDAC is not well understood. Here, we report that the splicing factor SRSF1 is highly expressed in pancreatitis, PDAC precursor lesions, and tumors. Increased SRSF1 is sufficient to induce pancreatitis and accelerate KRASG12D-mediated PDAC. Mechanistically, SRSF1 activates MAPK signaling—partly by upregulating interleukin 1 receptor type 1 (IL1R1) through alternative-splicing-regulated mRNA stability. Additionally, SRSF1 protein is destabilized through a negative feedback mechanism in phenotypically normal epithelial cells expressing KRASG12D in mouse pancreas and in pancreas organoids acutely expressing KRASG12D, buffering MAPK signaling and maintaining pancreas cell homeostasis. This negative feedback regulation of SRSF1 is overcome by hyperactive MYC, facilitating PDAC tumorigenesis. Our findings implicate SRSF1 in the etiology of pancreatitis and PDAC, and point to SRSF1-misregulated alternative splicing as a potential therapeutic target.
This article is highlighted and featured on the cover of the July issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Circadian Disruption and Colorectal Cancer Incidence in Black Women
Background: Animal and experimental studies suggest circadian disruption increases colorectal cancer risk, but evidence in humans is limited. We examined night shift work, chronotype, and residential position within a time zone, proxies for circadian disruption, in relation to colorectal cancer risk.
Methods: Participants in the Black Women’s Health Study, a prospective cohort of 59,000 Black American women established in 1995, reported history of night shift work and chronotype on follow-up questionnaires. Residential position within a time zone was estimated using participant addresses at each questionnaire cycle. Number of colorectal cancer cases and follow-up duration varied by analysis depending on timing of exposure assessment, ranging from 204 over the 2005 to 2018 night shift work study period to 452 over the 1995 to 2018 residential position study period. Cox proportional hazards regression was used to estimate multivariable-adjusted HRs and 95% confidence intervals (CI).
Results: Compared with never having worked a night shift, working a night shift for ≥10 years was associated with increased colorectal cancer risk (HR = 1.64; 95% CI, 1.01–2.66). However, shorter duration was not. The HR for evening versus morning chronotype was 0.96 (95% CI, 0.73–1.27). Westward position of residence within a time zone was not associated with colorectal cancer risk (HR per 5-degree longitude increase: 0.92; 95% CI, 0.82–1.03).
Conclusions: Our findings suggest a possible increased risk of colorectal cancer associated with long duration night shift work; however, results require confirmation in larger studies. Impact: Circadian disruption from long-term night shift work may contribute to colorectal cancer development in Black women.
This article is highlighted in the July issue.
Journal: Cancer Immunology Research
IFNγ signaling pathway defects are well-known mechanisms of resistance to immune checkpoint inhibitors. However, conflicting data have been reported, and the detailed mechanisms remain unclear. In this study, we have demonstrated that resistance to immune checkpoint inhibitors owing to IFNγ signaling pathway defects may be primarily caused by reduced MHC-I expression rather than by the loss of inhibitory effects on cellular proliferation or decreased chemokine production. In particular, we found that chemokines that recruit effector T cells were mainly produced by immune cells rather than cancer cells in the tumor microenvironment of a mouse model, with defects in IFNγ signaling pathways. Furthermore, we found a response to immune checkpoint inhibitors in a patient with JAK-negative head and neck squamous cell carcinoma whose HLA-I expression level was maintained. In addition, CRISPR screening to identify molecules associated with elevated MHC-I expression independent of IFNγ signaling pathways demonstrated that guanine nucleotide-binding protein subunit gamma 4 (GNG4) maintained MHC-I expression via the NF-κB signaling pathway. Our results indicate that patients with IFNγ signaling pathway defects are not always resistant to immune checkpoint inhibitors and highlight the importance of MHC-I expression among the pathways and the possibility of NF-κB–targeted therapies to overcome such resistance.
A commentary related to this article can be found here.
Journal: Cancer Prevention Research
Although the protective effects of physical activity against several cancers are well established, evidence is inconsistent concerning Asian populations. Therefore, we assessed the association between the characteristics of physical activity and overall and type-specific cancer incidence in Koreans and examined the differences in association according to obesity status. Using prospective data from 112,108 participants in the Health Examinees study-G from 2004 to 2013, we evaluated the association between leisure-time physical activity (LTPA) and the incidence of overall and type-specific cancers using the Cox proportional hazards model. Self-reported LTPA participation, duration per week, intensity, type, and diversity were assessed. The incidence of overall and type-specific cancers, including colorectal, gastric, lung, breast, and prostate cancer and 13 obesity-related cancers, was identified using the Korea Central Cancer Registry from 1999 to 2018. Analyses were also stratified according to obesity status. In overweight males, participation in vigorous LTPA [HR, 0.84; 95% confidence interval (CI), 0.72–0.97] and walking (HR, 0.84; 95% CI, 0.72–0.98) were associated with a lower risk of cancer overall. Regarding cancer types, climbing was marginally associated with a lower risk of colorectal cancer in overweight males (HR, 0.61; 95% CI, 0.37–1.00). In normal-weight females, although there was an increased risk in those performing recreational activities, this risk was attenuated when those diagnosed with thyroid cancer were excluded. In the analysis for 13 obesity-related cancers, consistent associations were found. These findings suggest the need for greater public awareness regarding physical activity among overweight individuals within the Asian population.
Journal: Cancer Research (July 1 issue)
Periostin+ Stromal Cells Guide Lymphovascular Invasion by Cancer Cells
Cancer cell dissemination to sentinel lymph nodes is associated with poor patient outcomes, particularly in breast cancer. The process by which cancer cells egress from the primary tumor upon interfacing with the lymphatic vasculature is complex and driven by dynamic interactions between cancer cells and stromal cells, including cancer-associated fibroblasts (CAF). The matricellular protein periostin can distinguish CAF subtypes in breast cancer and is associated with increased desmoplasia and disease recurrence in patients. However, as periostin is secreted, periostin-expressing CAFs are difficult to characterize in situ, limiting our understanding of their specific contribution to cancer progression. Here, we used in vivo genetic labeling and ablation to lineage trace periostin+ cells and characterize their functions during tumor growth and metastasis. Periostin-expressing CAFs were spatially found at periductal and perivascular margins, were enriched at lymphatic vessel peripheries, and were differentially activated by highly metastatic cancer cells versus poorly metastatic counterparts. Surprisingly, genetically depleting periostin+ CAFs slightly accelerated primary tumor growth but impaired intratumoral collagen organization and inhibited lymphatic, but not lung, metastases. Periostin ablation in CAFs impaired their ability to deposit aligned collagen matrices and inhibited cancer cell invasion through collagen and across lymphatic endothelial cell monolayers. Thus, highly metastatic cancer cells mobilize periostin-expressing CAFs in the primary tumor site that promote collagen remodeling and collective cell invasion within lymphatic vessels and ultimately to sentinel lymph nodes.
This article is featured on the cover of the July 1 issue.
Journal: Cancer Research (July 15 issue)
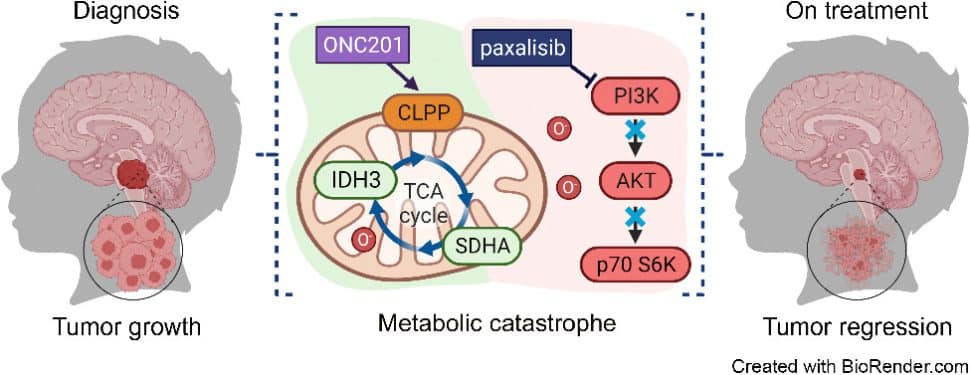
ONC201 in Combination with Paxalisib for the Treatment of H3K27-Altered Diffuse Midline Glioma
Diffuse midline gliomas (DMG), including diffuse intrinsic pontine gliomas (DIPG), are the most lethal of childhood cancers. Palliative radiotherapy is the only established treatment, with median patient survival of 9 to 11 months. ONC201 is a DRD2 antagonist and ClpP agonist that has shown preclinical and emerging clinical efficacy in DMG. However, further work is needed to identify the mechanisms of response of DIPGs to ONC201 treatment and to determine whether recurring genomic features influence response. Using a systems-biological approach, we showed that ONC201 elicits potent agonism of the mitochondrial protease ClpP to drive proteolysis of electron transport chain and tricarboxylic acid cycle proteins. DIPGs harboring PIK3CA mutations showed increased sensitivity to ONC201, whereas those harboring TP53 mutations were more resistant. Metabolic adaptation and reduced sensitivity to ONC201 was promoted by redox-activated PI3K/Akt signaling, which could be counteracted using the brain penetrant PI3K/Akt inhibitor, paxalisib. Together, these discoveries coupled with the powerful anti-DIPG/DMG pharmacokinetic and pharmacodynamic properties of ONC201 and paxalisib have provided the rationale for the ongoing DIPG/DMG phase II combination clinical trial NCT05009992.
This article is featured on the cover of the July 15 issue.
Journal: Clinical Cancer Research (July 1 issue)
Purpose: Devimistat (CPI-613) is a novel inhibitor of tumoral mitochondrial metabolism. We investigated the effect of devimistat in vitro and in a phase Ib clinical trial in patients with advanced biliary tract cancer (BTC). Patients and Methods: Cell viability assays of devimistat ± gemcitabine and cisplatin (GC) were performed and the effect of devimistat on mitochondrial respiration via oxygen consumption rate (OCR) was evaluated. A phase Ib/II trial was initiated in patients with untreated advanced BTC. In phase Ib, devimistat was infused over 2 hours in combination with GC on days 1 and 8 every 21 days with a primary objective to determine the recommended phase II dose (RP2D). Secondary objectives included safety, overall response rate (ORR), progression-free survival (PFS), and overall survival (OS). Results: In vitro, devimistat with GC had a synergistic effect on two cell lines. Devimistat significantly decreased OCR at higher doses and in arms with divided dosing. In the phase Ib trial, 20 patients received a median of nine cycles (range, 3–19). One DLT was observed, and the RP2D of devimistat was determined to be 2,000 mg/m2 in combination with GC. Most common grade 3 toxicities included neutropenia (n = 11, 55%), anemia (n = 4, 20%), and infection (n = 3, 15%). There were no grade 4 toxicities. After a median follow-up of 15.6 months, ORR was 45% and median PFS was 10 months (95% confidence interval, 7.1–14.9). Median OS is not yet estimable. Conclusions: Devimistat in combination with GC is well tolerated and has an acceptable safety profile in patients with untreated advanced BTC.
This article is highlighted in the July 1 issue.
Journal: Clinical Cancer Research (July 15 issue)
Purpose: To assess whether higher plasma 25-hydroxyvitamin D [25(OH)D] is associated with improved outcomes in colon cancer and whether circulating inflammatory cytokines mediate such association. Experimental Design: Plasma samples were collected from 1,437 patients with stage III colon cancer enrolled in a phase III randomized clinical trial (CALGB/SWOG 80702) from 2010 to 2015, who were followed until 2020. Cox regressions were used to examine associations between plasma 25(OH)D and disease-free survival (DFS), overall survival (OS), and time to recurrence (TTR). Mediation analysis was performed for circulating inflammatory biomarkers of C-reactive protein (CRP), IL6, and soluble TNF receptor 2 (sTNF-R2). Results: Vitamin D deficiency [25(OH)D <12 ng/mL] was present in 13% of total patients at baseline and in 32% of Black patients. Compared with deficiency, nondeficient vitamin D status (≥12 ng/mL) was significantly associated with improved DFS, OS, and TTR (all P log-rank<0.05), with multivariable-adjusted HRs of 0.68 (95% confidence interval, 0.51–0.92) for DFS, 0.57 (0.40–0.80) for OS, and 0.71 (0.52–0.98) for TTR. A U-shaped dose–response pattern was observed for DFS and OS (both P nonlinearity<0.05). The proportion of the association with survival that was mediated by sTNF-R2 was 10.6% (P mediation = 0.04) for DFS and 11.8% (P mediation = 0.05) for OS, whereas CRP and IL6 were not shown to be mediators. Plasma 25(OH)D was not associated with the occurrence of ≥ grade 2 adverse events. Conclusions: Nondeficient vitamin D is associated with improved outcomes in patients with stage III colon cancer, largely independent of circulation inflammations. A randomized trial is warranted to elucidate whether adjuvant vitamin D supplementation improves patient outcomes.
This article is highlighted in the July 15 issue.
Journal: Molecular Cancer Research
Mutations in Fms-like tyrosine kinase 3 (FLT3) are common drivers in acute myeloid leukemia (AML) yet FLT3 inhibitors only provide modest clinical benefit. Prior work has shown that inhibitors of lysine-specific demethylase 1 (LSD1) enhance kinase inhibitor activity in AML. Here we show that combined LSD1 and FLT3 inhibition induces synergistic cell death in FLT3-mutant AML. Multi-omic profiling revealed that the drug combination disrupts STAT5, LSD1, and GFI1 binding at the MYC blood superenhancer, suppressing superenhancer accessibility as well as MYC expression and activity. The drug combination simultaneously results in the accumulation of repressive H3K9me1 methylation, an LSD1 substrate, at MYC target genes. We validated these findings in 72 primary AML samples with nearly every sample demonstrating synergistic responses to the drug combination. Collectively, these studies reveal how epigenetic therapies augment the activity of kinase inhibitors in FLT3-ITD (internal tandem duplication) AML.
This article is highlighted in the July issue.
Journal: Molecular Cancer Therapeutics
Arginase 1/2 Inhibitor OATD-02: From Discovery to First-in-man Setup in Cancer Immunotherapy
Pharmacologic inhibition of the controlling immunity pathway enzymes arginases 1 and 2 (ARG1 and ARG2) is a promising strategy for cancer immunotherapy. Here, we report the discovery and development of OATD-02, an orally bioavailable, potent arginases inhibitor. The unique pharmacologic properties of OATD-02 are evidenced by targeting intracellular ARG1 and ARG2, as well as long drug-target residence time, moderate to high volume of distribution, and low clearance, which may jointly provide a weapon against arginase-related tumor immunosuppression and ARG2-dependent tumor cell growth. OATD-02 monotherapy had an antitumor effect in multiple tumor models and enhanced efficacy of the other immunomodulators. Completed nonclinical studies and human pharmacokinetic predictions indicate a feasible therapeutic window and allow for proposing a dose range for the first-in-human clinical study in patients with cancer.
This article is highlighted and featured on the cover of the July issue.
Journal: Cancer Research Communications
Artificial intelligence (AI) and machine learning (ML) are becoming critical in developing and deploying personalized medicine and targeted clinical trials. Recent advances in ML have enabled the integration of wider ranges of data including both medical records and imaging (radiomics). However, the development of prognostic models is complex as no modeling strategy is universally superior to others and validation of developed models requires large and diverse datasets to demonstrate that prognostic models developed (regardless of method) from one dataset are applicable to other datasets both internally and externally. Using a retrospective dataset of 2,552 patients from a single institution and a strict evaluation framework that included external validation on three external patient cohorts (873 patients), we crowdsourced the development of ML models to predict overall survival in head and neck cancer (HNC) using electronic medical records (EMR) and pretreatment radiological images. To assess the relative contributions of radiomics in predicting HNC prognosis, we compared 12 different models using imaging and/or EMR data. The model with the highest accuracy used multitask learning on clinical data and tumor volume, achieving high prognostic accuracy for 2-year and lifetime survival prediction, outperforming models relying on clinical data only, engineered radiomics, or complex deep neural network architecture. However, when we attempted to extend the best performing models from this large training dataset to other institutions, we observed significant reductions in the performance of the model in those datasets, highlighting the importance of detailed population-based reporting for AI/ML model utility and stronger validation frameworks.
- We have developed highly prognostic models for overall survival in HNC using EMRs and pretreatment radiological images based on a large, retrospective dataset of 2,552 patients from our institution.
- Diverse ML approaches were used by independent investigators. The model with the highest accuracy used multitask learning on clinical data and tumor volume.
- External validation of the top three performing models on three datasets (873 patients) with significant differences in the distributions of clinical and demographic variables demonstrated significant decreases in model performance.



