Editors’ Picks, December 2025: Potent Molecular Glues, Personalized CAR T-cell Therapies, and More
The countdown to the new year is nearly upon us, but no need to count down to the last installment of Editors’ Picks in 2025 … because here it is! With articles on everything from a potent molecular glue for treating pancreatic cancer to a molecular framework for developing personalized CAR T-cell therapies, the editors of the 10 peer-reviewed journals published by the American Association for Cancer Research (AACR) are showing that we have a lot of exciting developments to look forward to in 2026.
Among the other studies you don’t want to miss before we wave goodbye to 2025 are methods to improve cervical cancer screening, ways to overcome resistance to PARP inhibitors used to treat ovarian cancer, and what we can learn from the gut microbiome and high-fat diets that may lead to strategies to prevent colorectal cancer.
As you prepare to question whether old acquaintance should be forgot, there’s one thing you should not forget: All of these articles are freely available for a limited time at the links provided with the abstracts below.
Journal: Blood Cancer Discovery
Timing Genomic Antigen Loss in Multiple Myeloma Treated with T Cell–Redirecting Immunotherapies
Genomic antigen loss is a recurring mechanism of resistance to chimeric antigen receptor T-cell (CAR-T) and T-cell engagers (TCE) in relapsed/refractory multiple myeloma (RRMM). Yet, it remains unclear whether these events are acquired under treatment or merely selected from preexisting, undetectable clones. By leveraging chemotherapy mutational signatures as temporal barcodes within whole-genome sequencing data, we could time genomic antigen escape in 4 of 11 patients with RRMM. In all cases, the biallelic loss was driven by genomic events acquired after exposure to BCMA- and GPCR5D-targeted CAR-T/TCE and not present at baseline. Longitudinal digital PCR analysis corroborated that resistance mutations were undetectable at therapy initiation but emerged preceding relapse. Among 752 newly diagnosed patients, only 2.7% and 9% had monoallelic inactivation of TNFRSF17 and GPCR5D, respectively, with no biallelic loss. Our findings suggest limited utility of mutational screening prior to CAR-T/TCE while underscoring the importance of dynamic surveillance during therapy.
Significance: Multiple myeloma has been demonstrated to recurrently develop resistance to T-cell redirection via genomic antigen escape. By leveraging chemotherapy mutational signatures, we demonstrate that somatic antigen-escape mechanisms are uniformly acquired following treatment initiation and not selected from among preexisting clones, emphasizing the importance of dynamic longitudinal surveillance for their emergence.
A related commentary was published in the November issue where this article was highlighted and was one of two studies featured on the cover. The other article was an editor’s pick last month.
Journal: Cancer Discovery
Cancer cells are acutely dependent on nuclear transport due to elevated transcriptional activity, suggesting an unrealized opportunity for selective therapeutic inhibition of the nuclear pore complex (NPC). Through large-scale phenotypic profiling of cancer cell lines, genome-scale functional genomic modifier screens, and mass spectrometry–based proteomics, we discovered that the clinical drug PRLX-93936 is a molecular glue that binds and reprograms the TRIM21 ubiquitin ligase to degrade the NPC. Upon compound-induced TRIM21 recruitment, the nuclear pore is ubiquitylated and degraded, resulting in the loss of short-lived cytoplasmic mRNA transcripts and the induction of cancer cell apoptosis. Direct compound binding to TRIM21 was confirmed via surface plasmon resonance and X-ray crystallography, whereas compound-induced TRIM21–nucleoporin complex formation was demonstrated through multiple orthogonal approaches in cells and in vitro. Phenotype-guided optimization yielded compounds with 10-fold greater potency and drug-like properties, along with robust pharmacokinetics and efficacy against pancreatic cancer xenografts and patient-derived organoids.
Significance: This study establishes the cancer therapeutic potential of optimized TRIM21 molecular glues to degrade the NPC and underscores the value of reexamining drugs with previously unknown mechanisms using current technologies.
This article was highlighted in and featured on the cover of the December issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Background: Human papillomavirus testing on vaginal self-samples (VSS) has recently been offered in France as an option for women aged 30 to 65 years who are not regularly screened for cervical cancer. Human papillomavirus testing can also be performed on first-void urine.
Methods: The CapU4 study is a three-arm randomized controlled trial enrolling 14,997 women aged 30 to 65 years who had no screening test recorded for more than 4 years and who did not respond to an invitation letter 12 months prior. Women were allocated to two experimental arms [mailing of a VSS or a urine self-sampling (USS) kit] or to a control arm (invitation to visit a physician to collect a cervical specimen).
Results: A total of 13,061 women were included. The intention-to-treat analysis demonstrated that the participation rate increased in the self-sampling arms (USS: 23.6%; VSS: 23.5%) compared with the control arm (12.9%). The per-protocol analysis did not show a favorable effect (USS: 11.1%; VSS: 12.6%), particularly for USS.
Conclusions: Invitations including VSS or USS kits increased participation in cervical cancer screening by approximately 11%. Half of the responding women in the self-sampling arms visited a physician to take a cervical specimen.
Impact: There is evidence that sending VSS kits can increase attendance at cervical cancer screening. However, no data exist suggesting that sending urine collection kits may also be effective in triggering participation compared with conventional invitation letters. The results of the CapU4 trial may generate innovative tools that could help optimize attendance at cervical cancer screening.
This article was highlighted in the December issue.
Journal: Cancer Immunology Research
Immune checkpoint blockade for the treatment of malignancies has been focused on reversing inhibitory pathways in T lymphocytes. NK cells are a potent innate defense against tumors and virally infected cells, but their therapeutic manipulation for anticancer immunity has been inadequately explored. Considerable attention has been focused on approaches to blocking inhibitory receptors on NK and myeloid cells. Most effort has been directed to the killer immunoglobulin-like receptors and CD94/NKG2A on NK cells. Another set of receptors with similar function in both NK cells and myeloid cells is the leukocyte immunoglobulin-like receptors (LILR) that interact with a wide variety of HLA molecules. Using pan–anti-HLA mAbs that recognize a conserved epitopic region on HLA also seen by LILRs, we investigated their functional effects in several models of tumor immunity. The pan–anti-HLA mAbs blocked the binding of most LILRs and did not block killer cell immunoglobulin-like receptors or CD94/NKG2A/C or T-cell receptor recognition. They also activated dysfunctional NK cells explanted from a variety of human cancers and resulted in enhancement of tumor immunity in humanized mice. The mAbs also exert direct antitumor effects. These results suggest that activation of innate immunity via disruption of HLA/LILR interactions is a potent approach for control of both primary tumors and potentially tumor metastases.
Journal: Cancer Prevention Research
This study examined trends in colorectal cancer screening modality utilization across the United States from 2016 to 2022, leveraging a large national claims database. The purpose was to identify national, regional, and demographic patterns in screening behavior during a period that encompassed the introduction of multitarget stool DNA testing (mt-sDNA) and the COVID-19 pandemic. Among 6.9 million colorectal cancer screenings analyzed, overall utilization increased through 2019, dipped in 2020 due to pandemic disruptions, and rebounded by 2022. Colonoscopy remained the dominant modality, with its utilization increasing in both relative and absolute terms. mt-sDNA testing experienced rapid adoption, increasing from less than 1% to 17% of all screenings, whereas fecal occult blood testing and fecal immunochemical testing declined. Multinomial logistic regression revealed that utilization patterns varied significantly by region, rurality, sex, age, and year. The Midwest and rural patients exhibited higher uptake of both colonoscopy and mt-sDNA compared with other groups, whereas the West maintained the highest reliance on fecal occult blood testing and fecal immunochemical testing. Findings highlight the nonuniform adoption of screening modalities across regions, urban and rural patients, categories of sex, and age cohorts. Understanding these patterns can inform and improve future resource allocation with the goal of increasing colorectal cancer screening uptake and adherence.
Prevention Relevance: This study informs cancer prevention by revealing regional and demographic variation in colorectal cancer screening modality use. Understanding these evolving patterns can inform and improve targeted strategies and allocation of resources to improve screening uptake and adherence.
This article was featured on the cover of the December issue.
Journal: Cancer Research (December 1 issue)
High-fat diet (HFD) is positively correlated with colorectal cancer, but there are notable interindividual differences in susceptibility to the tumor-promoting effects of HFD. A better understanding of the mechanisms that modulate the outcomes of HFD could help inform precision prevention strategies for colorectal cancer. In this study, we found a key role for the gut microbiota in the individual differences observed in the tumor-promoting effects of HFD. Analysis of the gut bacteria enriched in mice resistant to HFD-induced cancer identified Lactobacillus johnsonii as an effective protector. Colonization of L. johnsonii increased intestinal chenodeoxycholic acid (CDCA) concentrations in HFD-exposed mice, which decelerated HFD-induced colorectal cancer progression. Mechanistically, L. johnsonii converted conjugated bile acid to CDCA via bile salt hydrolase, and CDCA induced mitochondrial dysfunction and oxidative stress to promote apoptosis, effectively suppressing tumor development. These results establish the gut microbiota as a mediator of interindividual differences in cancer susceptibility induced by HFD and reveal a probiotic strategy with the ability to inhibit tumorigenesis, suggesting a possible route to reduce HFD-induced colorectal cancer progression.
Significance: Investigation of bacteria–host interactions that alter cancer susceptibility uncovers suppression of high-fat diet–induced colorectal cancer by Lactobacillus johnsonii, offering a translational approach to improve cancer prevention.
A related commentary was published in the December 1 issue.
Journal: Cancer Research (December 15 issue)
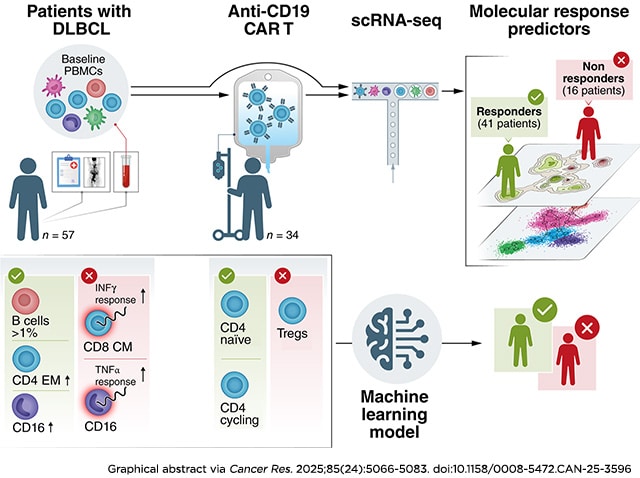
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment landscape for relapsed/refractory B-cell malignancies. Despite its success, approximately 60% of patients experience treatment failure, underscoring the need to better understand the determinants of response and resistance. We performed single-cell RNA sequencing of pretreatment peripheral blood samples and anti-CD19 CAR T-cell products from 57 diffuse large B-cell lymphomas (DLBCL), correlating molecular and cellular features with clinical outcomes. At the time of leukapheresis, responders presented elevated levels of CD16+ monocytes and CD4+ effector memory T cells. In contrast, nonresponders showed an inflammation-driven gene expression signature across T-cell and myeloid compartments, marked by upregulation of TNFα response signaling pathways. Notably, the presence of malignant or healthy B cells (13 of 57 patients) was strongly associated with a favorable response. These findings shed light on the immune landscape conducive to successful CAR T-cell therapy and offer a molecular framework for developing personalized tools to improve patient selection, stratification, and the design of next-generation CAR T-cell treatments.
Significance: Single-cell analysis of pretreatment peripheral blood from diffuse large B-cell lymphoma identified immune cell populations and genetic signatures correlated with CAR T-cell outcomes, informing patient selection, treatment strategies, and next-generation CAR T development.
Journal: Clinical Cancer Research (December 1 issue)
Purpose: Activating mutations in AKT genes are rare but play an important role in the commonly dysregulated PI3K/AKT/mTOR signaling pathway in multiple cancers. NCI-MATCH (EAY131) is a tumor-agnostic platform trial that enrolled patients to targeted therapies based on matching tumor genomic alterations. Subprotocol Z1K evaluated ipatasertib, a pan-AKT inhibitor, in patients with AKT1E17K-mutant metastatic tumors.
Patients and Methods: Patients received ipatasertib 400 mg orally once daily in a 28-day cycle until progression or unacceptable toxicity. Patients with well-controlled diabetes were eligible. Patients with known KRAS, NRAS, HRAS, or BRAF mutations were excluded. Prior PI3K and mTOR inhibitors were allowed. Prior AKT inhibitors were excluded. The primary endpoint was objective response rate (ORR). Secondary endpoints included progression-free survival, 6-month progression-free survival, and toxicity.
Results: Thirty-five patients were enrolled, and 29 patients were included in the prespecified primary efficacy analysis. Multiple histologies were enrolled, with breast (n = 18) and gynecologic (n = 7) being the most common. The majority had >3 lines of prior therapy (19/29; 65.5%). The ORR was 24.1% (7/29; 90% confidence interval, 11.9%–40.6%) with P < 0.001 against a null rate of 5%. All responses were partial responses. The median response duration was 10.1 months (90% confidence interval, 3.7–10.8). The most common toxicities of any grade included diarrhea (n = 25), nausea (n = 13), and hyperglycemia (n = 9). Grade 3/4 toxicities observed were consistent with reported toxicities for AKT inhibition. Twelve grade 3 events occurred that were thought to be at least possibly related to treatment.
Conclusions: The study met its primary endpoint with an ORR of 24.1% (P < 0.001), with ipatasertib demonstrating clinically significant activity in heavily pretreated patients with various tumors harboring AKT1E17K mutations.
A related commentary was published in the December 1 issue.
Journal: Clinical Cancer Research (December 15 issue)
Purpose: This phase Ib dose-escalation/expansion trial (NCT03400176) enrolled patients with CLL who did not achieve a complete response (CR) with ibrutinib or had developed resistance mutations. Ianalumab (VAY736), an anti–B cell–activating factor receptor monoclonal antibody, combined with ibrutinib significantly improved survival and reduced tumor burden in preclinical chronic lymphocytic leukemia (CLL) models.
Patients and Methods: Patients received intravenous ianalumab (escalation: 0.3–9.0 mg/kg; expansion: 3.0 mg/kg) once every 2 weeks and continued ibrutinib (420 mg) once daily for up to eight cycles of 28 days. The study aimed to evaluate the safety, tolerability, recommended dose, and antitumor activity of this combination.
Results: Thirty-nine patients were treated (escalation: n = 15; expansion: n = 24). No dose-limiting toxicities were observed. Of the 39 patients, 38.5% were in CR or CR with incomplete marrow recovery at cycle 9 (C9). At C9 day 1, 17 patients (43.6%) achieved undetectable measurable residual disease in blood or bone marrow. Grade ≥3 adverse events occurred in 16 patients (41.0%), which were treatment-related in nine (23.1%). No on-treatment deaths were reported; one patient died because of COVID-19 during the posttreatment period. Seventeen patients (43.6%) discontinued ibrutinib at or after C9 day 1 and remained off therapy for 12.1 to 24.5 months. Preliminary RNA sequencing and flow cytometry data support both NK- and T-cell activation with ianalumab.
Conclusions: The combination was well tolerated, with 43.6% of patients discontinuing ibrutinib therapy. Biomarker data suggest that ianalumab increased NK- and T-cell activation. These data support further evaluations of ianalumab in combination with Bruton tyrosine kinase inhibitors for patients with CLL.
Journal: Molecular Cancer Research
In ovarian cancer, resistance to conventional treatments has prompted the search for alternative targets and/or cells within the tumor microenvironment that could enhance tumor cell death. Ferroptosis, an iron-dependent, lipid peroxide–triggered form of cell death, is one such pathway. Cancer-associated fibroblasts (CAF) are key stromal cells in the ovarian tumor microenvironment that can affect therapeutic responses. Using various genetic approaches, we generated multiple DDR2-expressing and DDR2-deficient human ovarian tumor and mouse breast tumor CAFs. We found that DDR2 expression in CAFs protects these cells from ferroptosis by regulating the xCT–GSH–GPX4 antioxidant pathway and cellular iron metabolism. Specifically, DDR2 regulates xCT expression through noncanonical p62-dependent NRF2 activation and the labile iron pool by controlling ferritinophagy. CAFs secrete factors, in a DDR2-dependent manner, that provide protection to ovarian tumor cells against olaparib-induced cell death, a clinically relevant PARP inhibitor (PARPi). Finally, we found that high expression of DDR2 in the stromal cells of human ovarian tumors is associated with poor response to PARPi in clinical trials. These findings suggest that ferroptotic regulation by DDR2 in ovarian tumor CAFs could affect therapeutic sensitivity and resistance to PARPi.
Implications: The action of the collagen receptor tyrosine kinase DDR2 in CAFs confers PARPi protection to ovarian tumor cells by protecting CAFs from ferroptosis.
This article was highlighted in the December issue. Read more about cancer cells and iron in this blog post.

Journal: Molecular Cancer Therapeutics
CanAg (CA242) is a carbohydrate antigen highly overexpressed in most gastrointestinal cancers, with minimal expression in normal tissue, making it an attractive target for antibody–drug conjugate (ADC) therapeutics in these cancers. Previous efforts to target CanAg with ADCs have shown limited clinical efficacy, possibly due to resistance to the tubulin inhibitor payloads used. IKS04 is a novel CanAg-targeting ADC comprising an anti-CanAg humanized mAb Isumab04 and a highly potent pyrrolobenzodiazepine prodrug payload. However, the use of potent payloads such as pyrrolobenzodiazepines can limit the maximum tolerated dose of ADCs, which in turn limits tumor tissue penetration and efficacy, particularly for high-expression targets such as CanAg. Coadministration of unconjugated antibody can potentially improve tumor tissue penetration, resulting in increased ADC efficacy. In this study, we evaluated the impact of Isumab04 coadministration on the distribution and efficacy of IKS04 in human tumor xenograft mouse models with different CanAg expression levels. Although the addition of the Isumab04 antibody showed minimal impact on IKS04 cell–killing activity in vitro in cells with moderate and high CanAg expression, coadministration of Isumab04 with IKS04 improved tumor tissue distribution of the ADC in both tumor spheroids and in vivo tumor models. This improved distribution correlated with increased efficacy in vivo, in which increasing doses of unconjugated antibody resulted in greater efficacy until apparent tumor saturation was reached. These results support the use of antibody coadministration to improve the efficacy of ADCs targeting high-expression antigens with highly potent payloads.
This article was highlighted in and featured on the cover of the December issue.
Journal: Cancer Research Communications
Xevinapant is an orally bioavailable antagonist of select members of the inhibitor of apoptosis protein family. Despite promising phase II data, combining xevinapant with chemoradiotherapy (CRT) failed to improve outcomes in the phase III TrilynX trial when combined with CRT for locally advanced head and neck squamous cell cancer (SCCHN). In immunocompetent mouse models of SCCHN, xevinapant plus CRT maintained or improved locoregional control but in a CD8+ T cell–independent manner. On addition of xevinapant to CRT, the numbers of tumor-infiltrating cytotoxic CD8+ T cells and NK cells were reduced, with remaining CD8+ T cells characterized by PD-1hi CD38hi expression and Nr4a3 dynamics consistent with nonresponsiveness to antigenic restimulation. Furthermore, combination treatment significantly downregulated gene expression associated with immune-related pathways, increased levels of immunodysregulatory acute-phase proteins, and decreased levels of necroptosis mediator receptor-interacting protein kinase 3. Overall, xevinapant plus CRT has an immunosuppressive effect on the tumor-immune microenvironment, which may explain its lack of clinical benefit.
Significance: Despite hugely promising randomized phase II study data, combined CRT plus xevinapant failed in the TrilynX phase III clinical trial in locally advanced SCCHN. We show that adding xevinapant to chemoradiotherapy in vivo dysregulates antitumor lymphocyte function, acute-phase proteins, and cell death pathways, with net immunosuppressive effects on the tumor-immune microenvironment.