New FDA-approved Therapeutic Improves Survival for Patients With Liposarcoma
Late last week, the U.S. Food and Drug Administration (FDA) approved a new treatment for one of the most common types of soft tissue sarcoma, liposarcoma. The therapeutic, eribulin mesylate (Halaven), was specifically approved for treating patients with liposarcoma that cannot be removed by surgery or that is advanced and whose cancer has progressed despite treatment with a chemotherapy regimen that includes an anthracycline such as doxorubicin.
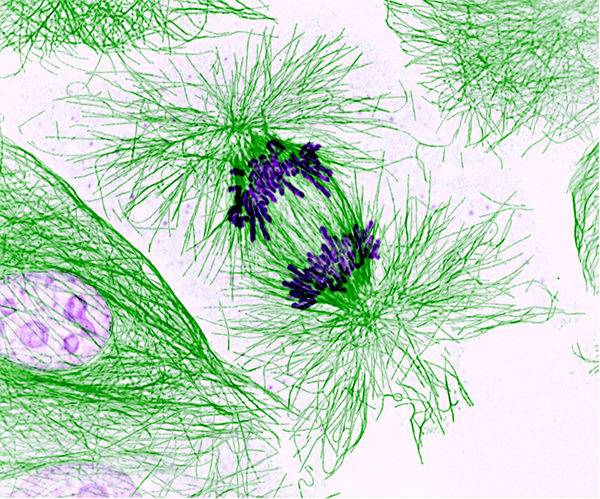
Dividing Pig Cell. Microtubules (green) are the target of eribulin mesylate, and prolonged inhibition of microtubule dynamics by the chemotherapeutic prevents cell division and ultimately leads to apoptosis. Image courtesy of the National Heart, Lung, and Blood Institute, National Institutes of Health.
In announcing the approval, Richard Pazdur, MD, director of the Office of Hematology and Oncology Products at the FDA said, “Halaven is the first drug approved for patients with liposarcoma that has demonstrated an improvement in [overall] survival time.”
Almost 12,000 people in the United States are estimated to have been newly diagnosed with a soft tissue sarcoma in 2015. These cancers arise in soft tissues of the body such as the muscles, tendons, fat, blood vessels, lymph vessels, nerves, and tissues around joints. Liposarcoma refers to those soft tissue sarcomas that arise in fat cells in any part of the body, most commonly fat cells in the muscles of the limbs or in the abdomen.
Eribulin mesylate is a structurally complex synthetic chemotherapeutic that is an analogue of halichondrin B, a natural product isolated from the marine sponge Halichondria okadai. Research published a decade ago in the AACR journals Cancer Research and Molecular Cancer Therapeutics revealed that eribulin mesylate exerts its anticancer effects by interfering with the cellular process of microtubule growth, which is essential for the proliferation and division of cells, including cancer cells, and that this ultimately causes cancer cells to die by a process known as apoptosis.
According to the FDA announcement, the approval of eribulin mesylate for liposarcoma was based on clinical trial data showing that the median overall survival for patients treated with eribulin mesylate was more than seven months greater than for those treated with the chemotherapeutic agent dacarbazine (15.6 months compared with 8.4 months).
Eribulin mesylate is the second new chemotherapeutic for the treatment of liposarcoma to be approved by the FDA in the past four months. As I discussed in a previous post, trabectedin (Yondelis) was approved for treating certain patients with liposarcoma, as well as those with another type of soft tissue sarcoma, leiomyosarcoma, after it increased median progression-free survival.
Further advances against all forms of sarcoma will be powered by new scientific insights into sarcoma biology, and the presentations at the recent AACR-organized conference on The Basic Science of Sarcoma, held in Salt Lake City from Nov. 3–4, showed that researchers are making huge strides in this regard. Suzie Siegel, a survivor of metastatic leiomyosarcoma, attended the conference in the hopes of learning more about the future of sarcoma treatment; a summary of the studies Suzie found most inspiring can be found in a recent post she wrote on the Sarcoma Alliance blog.