Researchers Discuss Disruption to DNA Damage Control
Later this week, more than 200 researchers will convene in Montreal for a four-day conference on DNA Repair: Tumor Development and Therapeutic Response organized by the American Association for Cancer Research (AACR). Attendees from 25 countries will discuss new research that is providing novel insights into the role of DNA repair deficiencies in cancer development and how this knowledge can provide novel approaches to cancer treatment.
DNA Repair and Cancer: What We Know
The primary cause of cancer initiation and progression is the accumulation of mutations in the genetic material of a cell (its DNA) over time. Some mutations are inherited and are present in every cell from birth. Most mutations, however, are acquired during a person’s lifetime. These mutations arise because the integrity of a cell’s DNA is constantly under threat from errors that occur during cell multiplication and as a result of exposure to toxins, such as those in cigarette smoke, and mutagens, such as ultraviolet radiation from the sun.
Given the potentially devastating consequences of loss of DNA integrity, cells have evolved several interrelated pathways that they use to repair damaged DNA.
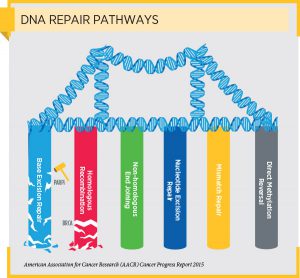
DNA Repair and Cancer: Harnessing Our Knowledge Therapeutically
Our current understanding of genetic deficiencies in DNA repair pathway genes is already being exploited therapeutically. For example, it is being used in the development of a class of anticancer therapeutics called poly ADP-ribose polymerase (PARP) inhibitors. PARP inhibitors work, at least in part, by blocking the base excision DNA repair function of PARP proteins. Cancer cells that have deficiencies in the homologous recombination DNA repair pathway, for example, ovarian cancer cells with certain BRCA1 or BRCA 2 mutations, are particularly susceptible to having their base excision DNA repair pathway blocked by a PARP inhibitor.
As discussed on this blog previously, one PARP inhibitor, olaparib (Lynparza), has been approved by the U.S. Food and Drug Administration (FDA) for women with advanced ovarian cancer associated with certain BRCA1 or BRCA 2 mutations. It has also been shown to be promising as a treatment for men with prostate cancer harboring DNA repair–pathway deficiencies.
Recent results from a phase III clinical trial testing another PARP inhibitor, niraparib, showed that it prolonged progression-free survival for patients with platinum-sensitive, recurrent ovarian cancer, with the greatest benefit being seen among those with tumors harboring BRCA1 or BRCA 2 mutations.
We hope that these results are just the tip of the iceberg for translating our understanding of DNA repair deficiencies in cancer into new approaches to treatment and that the AACR Special Conference on DNA Repair: Tumor Development and Therapeutic Response, which is being presented in association with the Radiation Science and Medicine Working Group of the AACR, will accelerate the pace of progress in this area.
The DNA Repair conference co-chairpersons are: Robert G. Bristow, MD, PhD, senior scientist at the University Health Network Princess Margaret Hospital in Toronto; Maria Jasin, PhD, member of the Developmental Biology Program and William E. Snee Chair at Memorial Sloan Kettering Cancer Center in New York; and Theodore S. Lawrence, MD PhD, professor and chair of the Department of Radiation Oncology at the University of Michigan, Ann Arbor. Lawrence is also chairperson (2015–2017) of the Radiation Science and Medicine Working Group of the AACR.



