Liquid Biopsy: Promises and Problems
Biopsies have been an integral part of cancer care for decades. In undiagnosed patients, oncologists can examine suspicious tissues and determine if the cells are benign or malignant. Following diagnosis, biopsies can reveal additional information about a patient’s tumor – What mutations are driving this cancer? Is this patient likely to respond to a particular therapy? In cases of cancer recurrence, biopsies can provide further information – Has this patient become resistant to treatment? Is the tumor driven by different mutations?
Though highly informative, traditional tumor biopsies have several drawbacks. Due to their inherently invasive nature, tumor biopsies can pose risks to the patient, such as bleeding and infection. Additionally, studies have shown that biopsies are not always representative of the entire tumor. Because many tumors are heterogeneous, different sites within the tumor can have different levels of gene expression and metastatic potential, and a tissue biopsy may not be indicative of all tumor phenotypes. Furthermore, some tumors are not easily accessible, and performing a biopsy may not be possible.
“Tumor biopsies can be risky, are sometimes unsuccessful, and are logistically not always feasible,” explained Geoffrey Oxnard, MD, a thoracic oncologist at Dana-Farber Cancer Institute and author of a recent paper in Clinical Cancer Research. “Today, we can use liquid biopsies as a shortcut when a tumor biopsy is needed for genotyping.”
Liquid biopsy, which is often performed using a simple blood draw, can tell scientists about cancer DNA in the blood. The DNA is sequenced in order to identify the genetic alterations in a patient’s tumor.
As discussed in a previous post, the first liquid biopsy test approved by the U.S. Food and Drug Administration (FDA) is used to identify patients with metastatic non-small cell lung cancer (NSCLC) who may benefit from targeted treatment. The presence of specific alterations to the EGFR gene determined through liquid biopsy identifies patients as candidates for treatment with erlotinib (Tarceva), a receptor tyrosine kinase inhibitor. While not FDA-approved, several other diagnostic tests exist, such as the Guardant360 blood test, which can analyze more than 70 genes across a wide variety of solid tumor types. Data from validation studies for this liquid biopsy test were recently published.
How does a liquid biopsy work?
The basic premise of analyzing cell-free DNA (cfDNA) is this: Cancer cells can shed DNA into the bloodstream through apoptosis, necrosis, and secretion. This DNA can be isolated and sequenced, revealing mutations and other changes to the genetic material, which can give scientists information about a patient’s cancer.
This premise, however, assumes that mutations found in the sequenced cfDNA comes solely from cancerous cells.
“When we study cfDNA using liquid biopsies, we can’t definitively say that we’re studying only the tumor in these samples. We are dipping our needle into the bloodstream and hoping to find scraps of tumor in there, but we’re just as likely to find mutations from all sorts of other sources,” said Oxnard.
These other sources can include fetal, viral, or bacterial DNA. Another key source of mutated DNA comes from white blood cells.
“What we haven’t quite realized is that many mutations detected via liquid biopsy are not derived just from cancer, but could also be benign mutations from the white blood cells,” noted Oxnard.
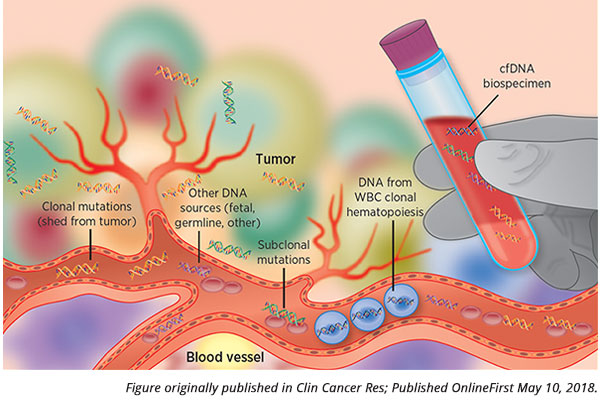
How do these benign mutations in white blood cells arise? The formation of new blood cells in the bone marrow starts from a single parental cell, which divides into a distinct subpopulation of cells. If the parental cell has an acquired benign genetic alteration, the entire subpopulation of cells will carry this alteration, as these cells are clonally derived from the parent. This phenomenon, known as clonal hematopoiesis of indeterminate potential (or often just clonal hematopoiesis), is common and occurs more frequently as we age.
“These white blood cell mutations end up contaminating liquid biopsy genotyping quite commonly,” said Oxnard. “This implies that we need to be cautious about how we use liquid biopsy technologies – if used improperly, we could get the wrong impression about a patient’s situation.”
How is cfDNA collected?
Following blood sample collection, the blood is spun down, separating the plasma from red and white blood cells and platelets, collectively called peripheral blood cells (PBCs). As cfDNA is found in the plasma, the researchers keep this portion for DNA isolation and sequencing, and discard the other cellular components of the blood.
However, because white blood cells also shed DNA into the bloodstream, mutations found in the plasma could stem from clonal hematopoiesis, in addition to mutations from the tumor. As an added wrinkle, since PBCs contain germline DNA, many diagnostic companies prefer to discard these cells, as germline testing often involves additional consent, noted Oxnard.
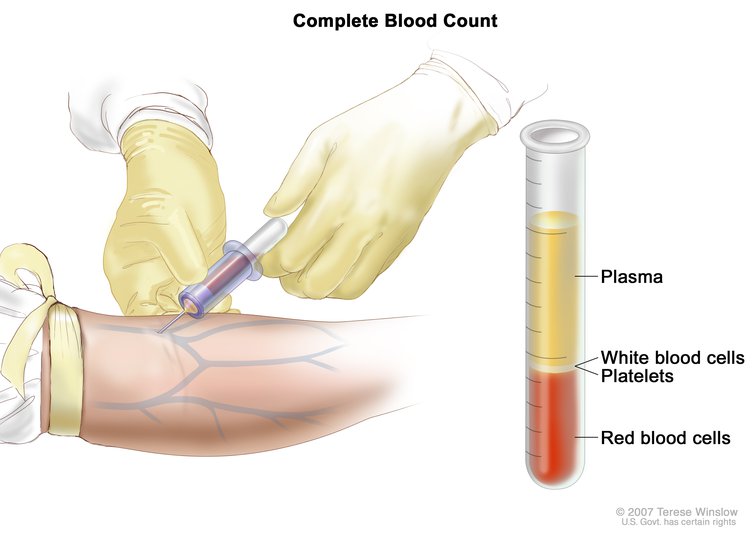
When performing a liquid biopsy, following a blood draw, the sample is spun down to separate the plasma from other components in the blood, allowing for the isolation of cfDNA. This image and the home page image are courtesy of the National Cancer Institute.
The established paradigm of liquid biopsy is that positive results – such as the detection of specific genetic mutations – could be acted upon, but a negative test would need to be reaffirmed by a tumor biopsy, Oxnard explained. “The sensitivity of the test isn’t perfect – if the tumor isn’t actively shedding DNA into the bloodstream, then you will get an erroneous negative result, a false negative,” he said.
“Our research describes an important twist on this paradigm,” he continued. “Our results show that sometimes a positive result can be unreliable as well.”
In their recent paper, Oxnard and colleagues found that the cfDNA isolated from patients with NSCLC harbored mutations in JAK2, TP53, and KRAS. By saving the patients’ PBCs, the researchers sequenced the DNA found in both the plasma and the PBCs and found that the mutations were often derived from clonal hematopoiesis, not from the patient’s tumor.
First, Oxnard and colleagues analyzed cfDNA and the accompanying PBCs from 58 patients with EGFR-mutant NSCLC through droplet digital PCR (ddPCR). Two of these patients harbored KRAS mutations, and both of these mutations stemmed from the PBCs. Next, the researchers utilized next-generation sequencing (NGS) to analyze cfDNA from 122 NSCLC patients. Five patients had JAK2 mutations that were derived from the PBCs; tumor samples available for three of these patients revealed no JAK2 mutations. Additionally, Oxnard and colleagues identified 108 TP53 mutations via plasma NGS. While 14 TP53 mutations were identified as tumor-derived variants, five were found in the PBCs and not in the tumor.
“TP53 mutations are common in lung cancer, but they are also common in white blood cells,” Oxnard explained. “If you find a TP53 mutation from a liquid biopsy blood test, based on our study, it’s hard to know if that DNA came from the tumor or from a white blood cell.”
KRAS mutations, which can have huge implications on cancer treatments, can be found in white blood cells as well, noted Oxnard. “Our data suggests that one out of 100 lung cancer patients’ liquid biopsy sample will have a KRAS mutation from their white blood cells, not from their cancer,” he said.
There are other genes commonly mutated in clonal hematopoiesis, such as DNMT3A, ASXL1, and TET2. As more research is performed, more genes associated with clonal hematopoiesis will be identified, noted Oxnard.
“Even though liquid biopsies are compelling, both negative and positive tests can be unreliable,” Oxnard continued. “This biospecimen can give us a lot of information, but we need to acknowledge its limitations as we use it clinically.”
In a commentary that accompanied Oxnard’s article, Joshua Bauml, MD, and Benjamin Levy, MD, agree that the findings by Oxnard and colleagues make the interpretation of cfDNA more complicated and add that more data are needed to understand the clinical implications of clonal hematopoiesis when utilizing liquid biopsies. They also note that fortunately, the majority of mutations associated with clonal hematopoiesis do not overlap with those found in solid tumors. As such, known molecular aberrations that may guide therapy for NSCLC, such as mutations in EGFR or ALK, have not been reported in clonal hematopoiesis and can be treated as clinically actionable should they be detected via liquid biopsy.
Liquid biopsies for cancer detection
“Mutations stemming from clonal hematopoiesis may pose a fundamental problem when considering the use of liquid biopsy for cancer detection,” Oxnard noted. “Our work would suggest that it’s hard to imagine developing a cancer detection test without sequencing the white blood cells.”
GRAIL, a diagnostic company that Oxnard collaborates with and consults for, sequences both the plasma and the white blood cells, with the goal of developing a blood test to detect cancer early.
From a single blood draw, the tests developed by GRAIL can analyze cfDNA through targeted sequencing, whole genome sequencing, and by measuring aberrant methylation patterns associated with cancer. For both targeted and whole genome sequencing assays, paired white blood cells are analyzed and compared with the cfDNA as a way to rule out false positives due to clonal hematopoiesis.
As recently presented at the AACR Annual Meeting 2018, blood tests developed by GRAIL were used to analyze blood samples from 878 participants with treatment-naïve, newly diagnosed cancer and 580 participants with no clinical cancer diagnosis. Data suggested the possibility of developing a test with a specificity greater than 99 percent. Additional data were reported at the American Society of Clinical Oncology (ASCO) meeting in June, supporting the development of a multicancer early-detection test.
An in-depth look at these recent data was recently reported by Matthew Herper in Forbes.
“From our prototype training assays, we found encouraging results that allowed us to detect the presence of cancer using three distinct methods with very few false positives,” said Alex Aravanis, MD, PhD in an AACR press release. “Taken together, this is a good first step toward the ultimate development of a blood-based early cancer detection test.”
Ongoing research is investigating the promises of liquid biopsy – for cancer detection, for cancer prognosis and monitoring, for prediction of response to therapy, and other applications. “The idea that we can learn about cancer from a blood test is compelling,” said Oxnard. “We are starting to push the boundaries of how we can use these liquid biopsy technologies in broader, greater, and more important ways.”
Interested in reading more about liquid biopsies? Check out this feature article by Stephen Ornes from Cancer Today magazine.




Well of course liquid biopsy has the potential to help manage NSCLC throughout all stages of lung cancer screening, MRD detection to guide adjuvant treatment, early detection of relapse, systemic treatment initiation & monitoring of response.
My sister runs a small clinic, and she would like to have a walk-in X-Ray scanner added in her facility, so it will be easier to conduct a biopsy. I’m not an expert in this that’s why I never knew that a liquid biopsy test is used to identify patients with metastatic. It’s also surprising to learn that cancer cells can shed DNA.