CRISPR in the Clinic: T-cell Genetic Engineering for Solid Tumors
Guest post by Emil Lou, MD, PhD

On October 7, 2020, the world awoke to news that the Royal Swedish Academy of Sciences had awarded this year’s Nobel Prize in Chemistry to Emmanuelle Charpentier, PhD, and Jennifer A. Doudna, PhD, both Fellows of the AACR Academy, for the discovery of the CRISPR/Cas9 “genetic scissors” that have revolutionized the field of gene editing. Over the past decade, this unusual-sounding mechanism has rolled off the tongues of scientists, physicians, and even the public, culminating in the announcement of this well-deserved award.
Cancer Research Catalyst writer Neha J. Pancholi, PhD, recently posted a very nice introduction to the CRISPR-Cas9 system. The post outlined the basic biology of CRISPR and explained how it is currently used in cancer research. For example, CRISPR/Cas9 is a highly efficient method for performing genetic screens that more quickly identify targetable mutations in cancer cells.
Beyond the lab, researchers are examining the safety and efficacy of CRISPR/Cas9 technology in humans. Editing immune cells to kill tumor cells may hold significant potential in the most difficult-to-treat forms of advanced cancers, for which current standards of care are purely palliative and not close to achieving cure.
As Pancholi noted, Carl June, MD, a pioneer in developing CAR (chimeric antigen receptor) T-cell therapy, has begun applying CRISPR/Cas9 to edit human cells for treatment. In February, June, along with colleague Edward Stadtmauer, MD, and colleagues, published the first report of CRISPR-engineered T-cell treatment of patients with cancer. In addition to treating two patients with myeloma with this protocol, they treated a patient with drug-resistant metastatic sarcoma, the first patient with a malignant solid tumor to be treated with this technology. The engineered T cells were able to recognize and destroy cancer cells in all three patients, and notably, none of the treated patients experienced any form of cytokine release syndrome.
This first-in-human trial was followed by reports from Hong Kong of a feasibility study using CRISPR-edited T cells in patients with refractory non-small cell lung cancer. This trial treated 12 patients with edited T cells and serves as the basis for future trials in this cancer. This establishment of safety of CRISPR in the clinical setting was heartening and well-received, but true solid tumor cancer efficacy, unfortunately, remains elusive.
Those two trials, and other ongoing trials in the field, provide context for a recently initiated human clinical trial harnessing the power of CRISPR/Cas9 to edit a novel intracellular checkpoint called cytokine-induced SH2 protein (CISH) to increase the capability of T cells to fight cancer in a human clinical setting. Anticancer activity data from targeting this novel intracellular checkpoint, in comparison to, and in combination with, targeting the well-known and readily druggable PD-1 checkpoint are provocative, but by virtue of being intracellular, CISH has been heretofore classically undruggable. Enter CRISPR:
Joint research carried out through collaboration between investigators from the National Cancer Institute, the University of Minnesota, and the sponsor of the trial, Intima Bioscience, provided pre- and peri-clinical data in solid tumors, which we are now translating into the clinic using CRISPR gene editing for patients with advanced gastrointestinal carcinomas.
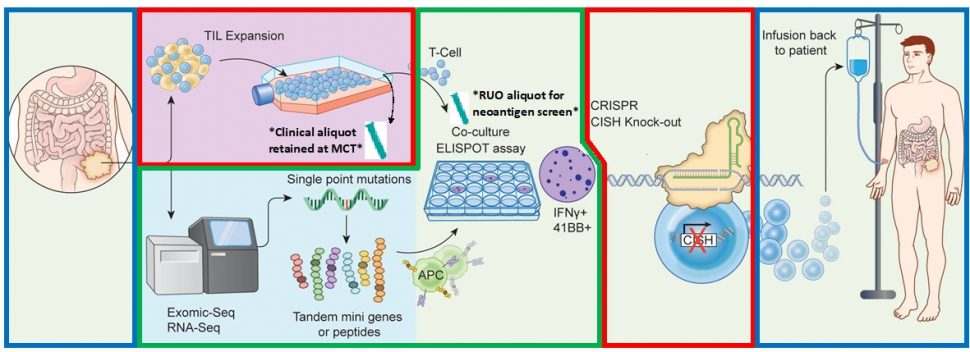
The COVID-19 pandemic created an additional hurdle to this long-anticipated trial; however, the trial has been actively recruiting since May and already has patients enrolled, with additional demand for access coming from patients nationally and internationally. Along with the complex science of CRISPR/Cas9 gene editing, numerous logistical factors make this trial a challenging clinical enterprise. For this reason, strong teamwork has been essential. We work in close partnership with the University of Minnesota’s Molecular and Cellular Therapeutics facility, one of only five such gene and cell therapy manufacturing centers in the country, to manufacture patient-specific CRISPR-engineered T cells. In addition, to effectively translate this cutting-edge technology from the laboratory, researchers who developed and characterized CISH as a novel immune checkpoint at our institution are an integral part of our trial team. Among them are Beau Webber, PhD, and Branden Moriarty, PhD, from the University of Minnesota’s Masonic Cancer Center.
The nuance of neutralizing an intracellular target that restricts antitumor immune response is not trivial, and highly precise CRISPR/Cas9-based genomic editing has been key to inactivating this target. With this achievement in hand, and despite the challenges of starting a clinical trial of this complexity in the middle of a pandemic, we felt an obligation to our patients to bring this novel and promising therapy to the armamentarium to fight solid tumor cancers as soon as possible. Given the potential importance of this novel checkpoint, coupled with precision CRISPR-engineered T-cell therapy, we hope that we can start to recapitulate the success that T-cell therapy and immuno-oncology have had, as shown by our colleagues in the field in liquid tumors, in the solid tumor arena, which has a significant unmet medical need.
Emil Lou is an associate professor of medicine at the University of Minnesota and medical director of the Clinical Trials Office – Solid Tumor Unit at the Masonic Cancer Center in Minneapolis.