Editors Share Picks from AACR Journals
As we move slowly toward longer days and warmer temperatures, we bring you the latest round of highlighted articles from the editors of the AACR journals. These articles include three clinical trials, a new drug targeting DNA damage response, the dynamics of small circular DNA fragments in cancer cells, and more. As always, these articles will be freely available online for a limited time, so check them out to learn more.
Journal: Clinical Cancer Research (February 1 issue)
A Phase II Study of the Efficacy and Safety of Oral Selinexor in Recurrent Glioblastoma
Glioblastoma (GBM) is the most common type of primary malignant brain tumors among adults. Increased expression of exportin-1 (XPO1), a nuclear protein that facilitates the transport of proteins from the nucleus to the cytoplasm, is found in solid tumors, including GBM, and correlates with poor outcome. Selinexor is a novel selective XPO1 inhibitor approved for the treatment of multiple myeloma and diffuse large B-cell lymphoma. This article reports the results of the open label phase II clinical trial, Efficacy and Safety of Selinexor (KPT-330) in Recurrent Glioblastoma (KING). The trial included 76 patients divided among different arms: a surgical arm, designed to study the intratumoral distribution of the drug, in which patients were given selinexor before undergoing surgery; and three medical arms that evaluated different dosing schedules in patients not undergoing surgery. The surgical arm showed adequate intratumoral drug penetration. In the medical arms, progression-free survival at six months was 10 percent in patients who received a selinexor dose of 50 mg/m2 twice weekly, 7.7 percent in those who received a 60 mg flat dose twice weekly, and 17 percent in those who received an 80 mg flat dose once per week. A reduction in tumor size was observed in 28 percent of patients, while the overall response rate per the Response Assessment in Neuro-Oncology (RANO) criteria was 8.8 percent, with one complete and two durable partial responses. Serious adverse events occurred in 34 percent of patients and were manageable with dose reduction, except in one case that was fatal. The study also included molecular analyses to identify a signature predictive of response. This article was highlighted in the February 1 issue.
Journal: Cancer Research (February 15 issue)
An in vivo CRISPR screen identifies stepwise genetic dependencies of metastatic progression
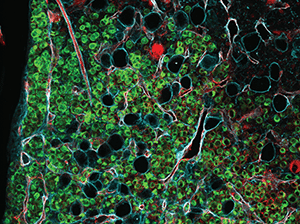
Cancer metastasis is the result of a multistep process that starts when tumor cells are shed from the primary or secondary tumors, enter the bloodstream and survive in the circulation. These circulating tumor cells (CTCs) then reach distant sites, where they extravasate and establish new tumors. The genetic events underlying this process are poorly characterized, partly due to the lack of models that faithfully recapitulate the human disease, making it difficult to develop therapies to prevent or treat metastatic cancer. In this study, the authors performed an in vivo loss-of-function CRISPR screen in newly derived xenografts obtained by transplanting CTCs from the blood of a patient with progressing breast cancer with metastasis to the bone, brain, liver, and lymph nodes. These models mirrored the patient’s metastatic disease and led to the identification of specific genes required for each step of the metastatic process. This “metastasis signature” was validated in patient-derived samples, confirming that high expression of the genes correlated with poor prognosis. Furthermore, treatment of the mouse models with inhibitors that target some of the genes impacting brain metastasis led to reduced tumor growth rate. The authors concluded that their findings provided new insights into the biology of metastasis and suggested novel drivers and treatment opportunities for metastatic breast cancers. This article was featured on the cover of the February 15 issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
As stool sampling for centralized colorectal cancer screening programs becomes more common, epidemiologists strive to use such programs to attain population-level data about the gut microbiome. However, the storage of fecal samples after collection, during colorectal cancer testing, and in transit between labs is not ideal for microbial preservation. In this study, the researchers stored fecal samples from 19 healthy participants using a variety of approved colorectal cancer screening kits from different countries, including tubes used for four fecal immunochemical tests (FIT) and two paper-based collection cards. Cards were stored at room temperature for seven days then frozen at -80 degrees Celsius or remained at room temperature until DNA extraction. Some FIT tubes were mailed to a FIT processing facility, underwent FIT testing, and were resealed and shipped to a microbial genomics lab. Others were stored at different temperatures to imitate transit during different seasons (4 degrees Celsius or 30 degrees Celsius for seven days, followed by 24 hours at room temperature, or seven days at room temperature). All tubes were then frozen at -80 degrees Celsius until processing. Collection cards exhibited high stability, even when left at room temperature for several weeks, as measured by their microbial diversity in comparison with control samples immediately frozen at -80 degrees Celsius. Most tubes adequately preserved samples, although significant variability was observed between genera, especially for samples stored at room temperature or 30 degrees Celsius. Processing of samples for FIT did not significantly affect stability. This study was highlighted and featured on the cover of the February issue.
Journal: Clinical Cancer Research (February 15 issue)
Despite the breadth of therapies targeting the growth factor receptor HER2—which drives the growth of several cancers, including some breast cancers—many tumors do not respond or develop treatment resistance. In this phase I clinical trial, the researchers tested KN026, a bispecific antibody targeting two different parts of the HER2 receptor, which showed preclinical efficacy against HER2-positive breast cancer models, including cells resistant to combination therapy with different HER2-targeting agents. They recruited 63 patients with metastatic, HER2-positive breast cancer and treated them with KN026 once weekly at 5 mg/kg or 10 mg/kg, once every two weeks at 20 mg/kg, or once every three weeks at 30 mg/kg. The researchers established 20 mg/kg and 30 mg/kg as the recommended phase II doses, which demonstrated a combined 28.1 percent overall response rate (14 partial responses and one complete response) and a median progression-free survival of 6.8 months. No dose-limiting toxicities were observed, and only 6.3 percent of patients experienced a grade 3 adverse event. Further, the researchers used a gene sequencing panel to evaluate potential biomarkers of KN026 response and identified CDK12, which is commonly co-amplified with HER2 in a variety of cancers. Patients with CDK12 amplifications had an overall response rate of 50 percent, as compared with 0 percent among patients without CDK12 amplifications. These data position KN026 as a candidate for future therapeutic development, especially in patients with HER2/CDK12 co-amplifications. This study was highlighted in the February 15 issue.
Journal: Molecular Cancer Research
Glioblastoma–Astrocyte Connexin 43 Gap Junctions Promote Tumor Invasion
Aggressive invasion into the surrounding tissue is a hallmark of GBM and reduces the success of surgical treatment, contributing to poor survival from the disease. Connexin 43 (Cx43) is the main gap junction protein in astrocytes, a type of glial cells in the central nervous system, and is also expressed in some GBM cells. This study assessed the role of Cx43 in tumor invasion and revealed that the protein plays dichotomous, cell-specific roles in GBM. While it has been established that Cx43 increases intercellular communication between GBM cells, resulting in tumor growth suppression, the authors found that GBM cell invasion increases upon co-culture with Cx43-expressing astrocytes and is significantly reduced in Cx43-deficient brains. Furthermore, an ex vivo slice culture model, in which GBM cell spheroids were implanted into mouse brain slices, demonstrated that Cx43 expression on GBM cells is localized at the tumor edge, suggesting Cx43 is redistributed to the periphery of primary tumors where it might drive invasion. Previous studies showed that GBM cells can manipulate the translational profiles of nearby cells by transferring repressive miRNA to modify their phenotypes. The authors found that, through a Cx43-dependent mechanism, GBM cells transferred miRNAs associated with cell–matrix adhesion to co-cultured astrocytes, suggesting GBM cells use miRNA to alter adhesion protein translation in surrounding astrocytes to promote an invasion-permissive environment. This article was highlighted and featured on the cover of the February issue.
Journal: Cancer Discovery
Cancer-promoting oncogenes are sometimes expressed from extrachromosomal DNA (ecDNA)—small, circular DNA elements that are not attached to chromosomes and segregate independently during cell division. The dynamics of how these ecDNAs behave in tumor cells, however, remains unknown. In this study, the researchers developed a CRISPR-based system called eTag, which fluorescently tags a breakpoint sequence found exclusively within ecDNAs, and used the system to visualize ecDNA molecules within the cell. They then used live cell imaging to study the dynamics of ecDNAs in a patient-derived glioblastoma neurosphere model, as well as a prostate cancer cell line, over the course of the cell cycle. In both models, daughter cells inherited widely variable numbers of ecDNAs during cell division. The ecDNAs clustered into “hubs” independent of DNA sequence, and those hubs frequently colocalized with nuclear bodies containing large amounts of RNA polymerase II, the primary polymerase responsible for messenger RNA transcription. These data help explain the mechanism by which ecDNAs promote the robust expression of oncogenes. A commentary on this study is available here.
Journal: Blood Cancer Discovery
Over 80 percent of pediatric patients with acute lymphoblastic leukemia (ALL) treated with the CAR T-therapy tisagenlecleucel (Kymirah) experience a complete remission, but around half of them eventually relapse. The current standard for predicting relapse is to monitor the levels of healthy B cells; because tisagenlecleucel depletes both healthy and cancerous B cells, recovery of healthy B cells would mean the CAR T-cell efficacy is waning. However, B-cell monitoring can miss some cases of resistance, as ALL can find other ways to grow in spite of the treatment. In this study, the researchers evaluated the use of liquid biopsy technologies to predict relapse in pediatric ALL patients from the ENSIGN and ELIANA clinical trials. They performed next-generation sequencing on fragments of tumor DNA found in the blood (ctDNA), and they performed flow cytometry—a technique that interrogates proteins on the cell surface—on circulating tumor cells. The researchers found that their next-generation sequencing approach was more sensitive than flow cytometry and highly accurate. All patients with detectable ctDNA six or 12 months after tisagenlecleucel treatment eventually relapsed, with a median lead time of 165 days from the time of ctDNA detection. The researchers suggested that earlier, more sensitive detection may enable physicians to escalate treatment before an overt relapse. This study was featured on the cover of the February issue, in an AACR press release, and in a related commentary.
Journal: Cancer Immunology Research
Many forms of immunotherapy improve the ability of CD8+ cytotoxic T lymphocytes (CTLs) to fight tumors. However, many patients do not respond to immunotherapy, and scientists have yet to determine what features differentiate responsive CTLs from non-responsive CTLs. In this study, the researchers harvested CTLs from pre- and post-treatment blood samples of four patients with advanced melanoma who were treated with an immune checkpoint inhibitor targeting PD-1. Two of the patients exhibited a complete response to anti-PD-1 therapy, and two of the patients experienced disease progression. The researchers performed single-cell RNA sequencing on the CTLs and found that the gene NKG7 was significantly upregulated during treatment in the cells of patients with a complete response but significantly downregulated during treatment in the cells of patients with progressive disease. They found that knocking down NKG7 expression in primary human CTLs impaired the secretion of cytotoxic granules, the toxic payloads that kill tumor cells, and decreased interaction between CTLs and tumor cells. Conversely, adding NKG7 mRNA to CTLs from patients whose disease progressed following anti-PD-1 therapy increased their cytotoxic ability and sensitized them to anti-PD-1 treatment in vitro. This study provides new insights into determinants of the antitumor immune response and positions NKG7 as a possible future biomarker and therapeutic target. A research article related to this study is available here.
Journal: Cancer Prevention Research
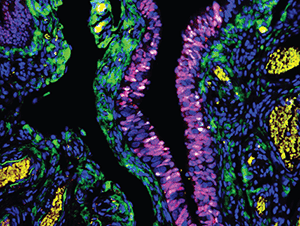
Most high-grade ovarian cancer is believed to begin in the fallopian tube, arising from lesions of serous tubal intraepithelial carcinoma (STIC) that progress into invasive carcinoma and infiltrate the ovary. Treatment with synthetic forms of progesterone, called progestins, has been shown to decrease ovarian cancer risk, but the mechanism remains unknown. In this study, the researchers used the mogp-TAg mouse model, which develops malignant lesions in the fallopian tube, to study the effects of progestin treatment on molecular changes in fallopian tube tissue. They treated the mice with a single dose of the progestin depo-medroxyprogesterone acetate (DMPA) and examined the fallopian tubes after three and seven weeks. By three weeks post-treatment, eight of 10 mice treated with a vehicle control had developed invasive carcinoma, compared with zero of ten DMPA-treated mice. Fallopian tubes from mice treated with DMPA had less staining of the proliferative marker Ki67, fewer clusters of mutant p53 cells, and fewer STIC lesions than mice treated with vehicle. The researchers observed increased caspase-3, a marker of apoptosis, in the fallopian tubes of mice treated short term with DMPA, specifically in clusters of p53-mutant cells, suggesting that DMPA promotes the death of malignant cells. These findings support the potential anticancer effects of progestins on the fallopian tube and characterize a previously unknown mechanism underlying these effects. This study was featured on the cover of the February issue.
Journal: Molecular Cancer Therapeutics
The DNA damage response (DDR) plays a critical role in cancer development and response to therapy. The ataxia telangiectasia mutated and Rad3-related (ATR) kinase, a major regulator of the DDR, is considered a promising target for anticancer drug development. RP-3500 is a novel oral ATR inhibitor under clinical evaluation in patients with advanced solid tumors with genetic alterations that render them sensitive to ATR inhibition. In this study, the authors described the structure of RP-3500 and showed that it is highly potent and selective in biochemical assays. The compound was highly effective as a monotherapy in several preclinical models with defects in BRCA1 or ATM pathway function. Importantly, an intermittent dosing schedule (three days on/four days off) reduced the hematologic toxicity (anemia) associated with continuous dosing and maximized tumor growth inhibition. In addition, concomitant intermittent, low-dose administration of the PARP inhibitors niraparib or olaparib and RP-3500 produced greater antitumor effects and was better tolerated than sequential administration. According to the authors, these results support the ongoing clinical evaluation of RP-3500 on an intermittent schedule as a monotherapy and in combination with low-dose PARP inhibitors. This article was highlighted and featured on the cover of the February issue.
Journal: Cancer Research (February 1 issue)
Chemotherapy remains the frontline treatment for most types of acute myelogenous leukemia (AML). Compacted chromatin associated with DNA methylation is more resistant to the DNA damaging effects of cytotoxic agents. In this study, the authors found that in AML cell lines and blasts from newly diagnosed patients the H3K27 histone methyltransferase enzyme EZH2 was recruited to the chromatin, and the repressive histone mark H3K27me3 rapidly accumulated on nascent DNA after replication. This observation provided the rational for combining EZH2 inhibition and chemotherapy to enhance DNA damage and apoptosis of leukemic cells. In cell lines, primary cells and AML mouse models, treatment with the EZH2 inhibitor GSK126 increased chromatin accessibility and enhanced DNA damage, apoptosis, and leukemia suppression induced by the cytotoxic drug doxorubicin. These effects were further enhanced in S-phase-enriched AML cells. In accordance with the transcriptional effect of inhibiting histone methylation, EZH2 inhibitor and doxorubicin cotreatment resulted in a partial transcriptional reprogramming and led to increased expression of genes that promote cell death and inhibit proliferation. According to the authors, combining chemotherapy with EZH2 inhibition for AML may represent a promising strategy to improve the efficacy of cytotoxic drugs and limit chemotherapy doses, which would in turn result in less severe side effects, especially in elderly patients and those with comorbidities. This article was highlighted in a commentary in the February 1 issue.



