Editors’ Picks, January 2026: Naked Mole Rat’s Cancer Resistance, the Impact of Tissue and Liquid Biopsy Concordance, and More
What does a naked mole rat, a nuclear exporter that “moonlights” as a chromatin regulator, and the combined power of tissue and liquid biopsy all have in common? They are all at the heart of innovative studies included in the year’s first edition of Editors’ Picks.

In one study, researchers found that the fact exportin-1 (XPO1) also acts as a chromatin regulator could make it a therapeutic target for treating a high-risk molecular subtype of acute myeloid leukemia. Another study examined why naked mole rats have a resistance to cancer, only to discover that they may actually have similar tumor initiation mechanisms to humans. Meanwhile, another study found that liquid and tissue biopsy worked better together in identifying actionable mutations that can be targeted with therapy.
Those are just a few of the studies highlighted this month by the editors of the 10 peer-reviewed journals published by the American Association for Cancer Research (AACR). Be sure not to miss any of them, and to read the full articles that are freely available for a limited time at the links provided with the abstracts below.
Journal: Blood Cancer Discovery
UBTF tandem duplications (UBTF-TD) define a high-risk molecular subtype of acute myeloid leukemia (AML). Although menin inhibitors show therapeutic promise in UBTF-TD AMLs, acquired resistance remains a challenge. In this study, we used proteomic, epigenetic, and functional analyses to uncover mechanisms underlying UBTF-TD leukemogenesis. Biochemical studies showed that UBTF-TDs result in structural destabilization and create nuclear export signal (NES) motifs, which mediate direct interactions with exportin-1 (XPO1). In cord blood CD34+ UBTF-TD models, these interactions were shown to drive aberrant chromatin binding and transcriptional activation of genes dysregulated in UBTF-TD tumors. Through mutagenesis, we demonstrated that these NES motifs are critical for localization of UBTF-TD proteins to chromatin, transcriptional dysregulation, and cellular proliferation and differentiation. In preclinical UBTF-TD models of human leukemia, we found that XPO1 inhibition disrupts UBTF-TD chromatin localization, reduces tumor burden, and promotes differentiation. These mechanistic findings highlight XPO1 inhibition as a potential therapy for UBTF-TD AMLs.
Significance: UBTF tandem duplications are a high-risk AML subtype. We identified a mechanism in which UBTF-TDs result in the generation of NES motifs, enabling aberrant interaction with XPO1. We found that XPO1 inhibitors block this interaction and impair leukemia growth, identifying a possible therapeutic strategy in UBTF-TD AML.
A related commentary was published in the January issue where this article was highlighted and was featured on the cover.
Journal: Cancer Discovery

There is growing interest in understanding the mechanisms underlying differences in cancer incidence among species (comparative oncology). The naked mole rat (NMR) is often referenced as “cancer resistant” and prior studies focused on identifying mechanisms explaining this. However, efforts to assess this in vivo have been limited. In this study, we provide evidence that the NMR presents as a novel autochthonous model of lung tumor initiation, driven by an introduction of the oncogenic Eml4-Alk fusion gene using CRISPR-mediated genome editing. Although in mice, the inversion alone is sufficient to drive tumorigenesis, the inversion alone was insufficient to drive tumorigenesis in the NMR lung, and tumor development required additional losses of the tumor suppressors p53 and pRb. Our findings suggest that the proposed “resistance” of the NMR to the development of cancer may reflect that the genetic events leading to tumor initiation are likely to be comparable with those present in human cells.
Significance: To identify evolutionary divergent mechanisms of cancer resistance, we assessed tumorigenesis in vivo using the NMR, a species considered to exhibit cancer resistance. Our findings suggest that the proposed “resistance” of NMRs to the development of cancer may reflect tumor initiation mechanisms comparable with the mechanisms present in humans.
A related commentary was published in the January issue where this article was highlighted and was featured on the cover.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Background: Preneoplastic lesions (PNL) represent a state intermediate between normal cells and invasive cancer. Individuals diagnosed with PNL are at increased risk of developing invasive cancer and represent a unique risk group. We estimated the proportion of invasive cancer that could be intercepted at multiple anatomic sites in individuals with PNL.
Methods: Using a literature survey of the prevalence, latency, and probability of progression of PNL to invasive cancer, we undertook a sensitivity analysis across proportions of invasive cancer from 5% to 95% that could be intercepted in a cohort of 100,000 individuals that would be avoided if PNL interception was successful. To calculate the number of avoidable invasive cancers, we used data that represent population-based, high-risk, unselected, and selected PNL case series and calculated the sensitivities and specificities of avoidable invasive cancers.
Results: Substantial invasive cancer reductions could be achieved by the interception of PNL that precede pancreas, gastric, bladder, prostate, breast, colorectal, and skin cancers. An intermediate reduction in cancer incidence was observed for lung and oral cancers. Limited impact on the global burden of cancer with the interception of PNL is likely for cervical, liver, and esophageal cancer, as well as myeloma and leukemia. Due to the estimates representing varying populations, the impacts of a specific PNL or cancer cannot be compared with another.
Conclusions: We identified substantial variation in the impact that the interception of PNL would have on the development of invasive cancer.
Impact: These results may guide research and implementation of studies intended to maximize the number of invasive cancers that can be avoided by the interception of PNL.
This article was highlighted in the January issue.
Journal: Cancer Immunology Research
T-cell Subset Features and Distributions Evolve across the Colorectal Precancer–Cancer Spectrum
The immune microenvironment is a crucial component of colorectal carcinoma that has been well characterized, but much less is known about the immune microenvironment of colorectal carcinoma precursors. We hypothesized that T-cell infiltrates might differ across the colorectal neoplastic spectrum. We leveraged the prospective cohort incident-tumor biobank method, which provided formalin-fixed, paraffin-embedded tumor tissue specimens (N = 1,825) from 790 colorectal carcinoma precursors (including hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, tubular adenomas, tubulovillous adenomas, and villous adenomas) and 1,035 colorectal carcinomas. We performed an in situ multispectral immunofluorescence assay for CD3, CD4, CD8, FOXP3 (negative, low, or high expression), PTPRC (CD45RO and CD45RA), MKI67 (Ki-67), and KRT (keratin) combined with supervised machine learning. CD3+CD4+ cells were more abundant than CD3+CD8+ cells in most precursors. In conventional adenomas, greater villous component correlated with fewer intraepithelial CD3+CD8+ cells. Serrated lesions, including hyperplastic polyps and sessile serrated lesions, exhibited higher densities of intraepithelial CD3+CD8+ cells compared with other precursors and carcinomas. Age strata of patients with precursors (including early-onset precursors) were not associated with differential T-cell infiltration patterns. Compared with invasive colorectal carcinoma, precursors generally showed higher densities of CD3+CD4+ cells and CD3+CD8+ cells with phenotypes of naive (CD45RA+CD45RO−), memory (CD45RA−CD45RO+), and regulatory (FOXP3+Low and FOXP3+High) in intraepithelial and lamina propria/stromal regions. In conclusion, T-cell infiltration patterns vary across different histopathologic types of the colorectal neoplastic spectrum from precursors to invasive carcinomas. Our findings shed light on how the tumor-immune microenvironment evolves during precursor development and progression to colorectal carcinoma.
This article was featured on the cover of the January issue.
Journal: Cancer Prevention Research
Aberrant DNA methylation and copy-number alterations (CNA) drive Barrett’s esophagus progression to esophageal adenocarcinoma; however, their combined utility for early detection is unclear. We aimed to identify and validate methylated DNA markers (MDM) and CNAs to distinguish esophageal adenocarcinoma/high-grade dysplasia (HGD) from nondysplastic Barrett’s esophagus (NDBE). In this multiphase, multicenter study, we discovered and validated MDMs and quantified CNAs utilizing whole-genome methylation sequencing of esophageal brushings. DNA biomarkers identified from discovery were further validated in independent patients with paired esophageal brushing and swallowed capsule sponge samples. MDMs were filtered against a reduced representation bisulfite sequencing dataset obtained from independent tissue samples to advance only concordant candidates. CNA burden was quantified using ichorCNA-derived aneuploidy scores (AS). Two hundred MDMs discovered in HGD (N = 18) and esophageal adenocarcinoma (N = 18) versus NDBE brushing samples (N = 18) were tested in independent samples (N = 146). A 52-MDM panel achieved a cross-validated AUC of 0.88 [95% confidence interval (CI), 0.82–0.95]; the addition of AS improved discrimination of HGD/esophageal adenocarcinoma from NDBE to 0.91 (95% CI, 0.86–0.97) AUC. At 80% specificity, the combined model detected 93% of esophageal adenocarcinoma and 88% of HGD cases. In paired capsule sponge samples, a 58-MDM panel achieved a cross-validated AUC of 0.77 (95% CI, 0.66–0.88); a combined 58-MDM and AS model achieved AUC 0.80 (95% CI, 0.7–0.9). MDMs and AS discerned HGD/esophageal adenocarcinoma from normal esophagus/NDBE in endoscopic brushing and capsule sponge samples. This approach may improve Barrett’s esophagus surveillance.
Prevention Relevance: This study demonstrates that combining epigenetic and genomic biomarkers across minimally invasive sampling methods can accurately distinguish HGD/esophageal adenocarcinoma from nondysplastic Barrett’s esophagus, offering promising, less invasive strategies to improve BE surveillance and enable endoscopic therapy for esophageal adenocarcinoma prevention and treatment.
This article was featured on the cover of the January issue.
Journal: Cancer Research (January 1 issue)
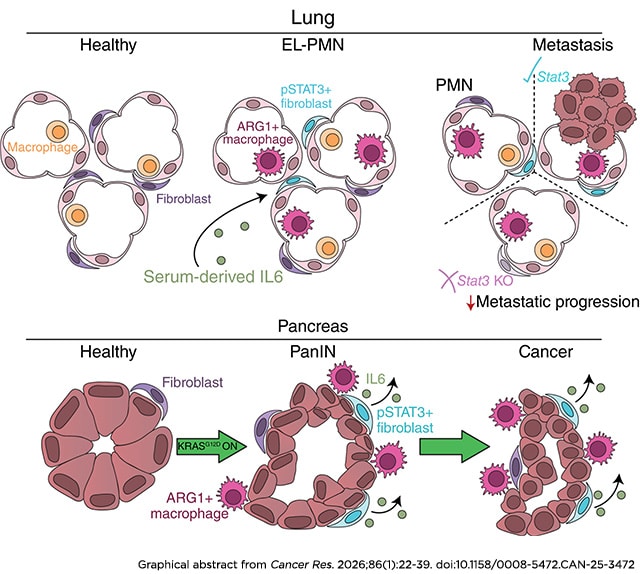
Fibroblast STAT3 Activation Drives Organ-Specific Premetastatic Niche Formation
Pancreatic cancer is associated with a high rate of metastasis and poor prognosis. The formation of a premetastatic niche (PMN) facilitates cancer cell spread and contributes to cancer mortality. Using murine pancreatic cancer models based on expression of oncogenic KRAS in the pancreas epithelium, we discovered that remodeling of the lung microenvironment occurred in mice bearing pancreatic precursor lesions prior to cancer formation. This early-lesion PMN resembled the PMN in cancer-bearing mice, and both feature characteristics of overt metastasis, such as transcriptional reprogramming, activation of fibroblast STAT3 signaling, and infiltration of immunosuppressive arginase 1–positive macrophages. Both patients with pancreatic cancer and mouse models demonstrated elevated serum IL6. Inactivating oncogenic KRAS reduced serum IL6 and reverted fibroblast STAT3 phosphorylation in mouse lungs; loss of lung fibroblast STAT3 phosphorylation was similarly observed when mice were treated with the pan-RAS inhibitor RMC-7977. Whereas arginase 1–positive macrophage infiltration was dispensable for fibroblast STAT3 activation, IL6 blockade inhibited lung fibroblast STAT3 activation. Functionally, fibroblast STAT3 activation was necessary for lung metastasis establishment and growth. Interestingly, activation of STAT3 in the PMN was present in the lungs but not in the liver, in which fibroblast reprogramming occurred only in overt metastasis, pointing to organ-specific PMN formation. In human metastasis samples, phosphorylated STAT3 in fibroblasts was similarly more abundant in the lungs than liver. Together, these data point to organ-specific mechanisms driving formation of the PMN and indicate that reprogramming of the microenvironment prior to metastasis might support early dissemination of pancreatic cancer.
Significance: Pancreatic oncogenic KRAS drives premetastatic niche formation in the lungs, but not liver, through fibroblast STAT3 activation to promote metastasis, indicating a lung-specific vulnerability for treating pancreatic cancer metastasis.
A related commentary was published in the January 1 issue.
Journal: Cancer Research (January 15 issue)
Small-molecule KRASG12C(OFF) inhibitors that bind to the inactive GDP-bound state of KRAS have demonstrated efficacy in patients with KRASG12C-mutant tumors, yet responses tend to be transient because of emergence of on-treatment resistance. Recently, RAS(ON) G12C–selective inhibitors, which bind to the active GTP-bound state of RAS, were described, and elironrasib is undergoing evaluation in multiple clinical trials. In this study, we generated resistant cell lines and patient-derived xenograft models to KRASG12C(OFF) and RAS(ON) G12C–selective inhibitors and interrogated resistance mechanisms using a multiomics strategy consisting of phosphoproteomics, whole-exome sequencing, and RNA sequencing combined with functional testing using small-molecule and CRISPR screens and RAS(ON) inhibitors being evaluated in clinical trials. Two models reactivated RAS signaling, either via KRASG12C gene amplification or NRASG13R mutation, and were vulnerable to dual inhibition by RAS(ON) G12C–selective and RAS(ON) multiselective inhibitors, RMC-4998 and RMC-7977. Two models, which lacked any discernable genomic alteration, acquired resistance associated with increased receptor tyrosine kinase activity and downstream persistent RAS activity and were sensitive to RAS-GTP inhibition by RMC-7977. Finally, one model displayed epithelial–mesenchymal transition, loss of RAS dependence, and acquired reliance on cell-cycle kinases and proteins associated with DNA damage response. This work highlights KRASG12C-selective inhibitor resistant states that parallel and complement clinical findings and demonstrate that a large subset could be overcome with a RAS(ON) multi-selective inhibitor as a stand-alone agent or in combination with other therapies.
Significance: Multi-omic characterization of resistance mechanisms to KRASG12C-selective inhibitors in non–small cell lung cancer provides insights that could inform precision medicine-based therapeutic approaches for improving the treatment of KRASG12C mutant tumors.
A related article was published in the January 15 issue.
Journal: Clinical Cancer Research (January 1 issue)
Purpose: This analysis evaluated the influence of tissue and liquid biopsy concordance on outcomes in patients enrolled in the ROME trial.
Patients and Methods: The ROME trial, a phase II multicenter study, enrolled 1,794 patients with advanced solid tumors. Next-generation sequencing was performed on tissue and liquid biopsies using FoundationOne CDx and FoundationOne Liquid CDx. A centralized molecular tumor board reviewed results to identify actionable alterations, with 400 patients randomly assigned to tailored therapy (TT) or standard-of-care groups. TT improved objective response rate and progression-free survival (PFS) in the intention-to-treat population. Concordance was defined as the detection of the same druggable alteration in both biopsy types; discordance indicated detection in only one.
Results: Concordance was present in 49% of cases, with alterations detected exclusively in tissue (35%) or liquid (16%) biopsies. Patients in the concordant group receiving TT experienced improved survival outcomes. The median overall survival was 11.05 versus 7.70 months in the standard-of-care group [HR = 0.74; 95% confidence interval, 0.51–1.07], and the median PFS was 4.93 versus 2.80 months (HR = 0.55; 95% confidence interval, 0.40–0.76), respectively. In contrast, the survival benefit of TT was less pronounced or absent in patients with discordant results. Overall survival was higher in the T + L group (11.05 months), followed by tissue-only (9.93 months) and liquid-only (4.05 months) groups. PFS followed a similar pattern, with the longest PFS in the T + L group (4.93 months) versus 3.06 months in tissue-only and 2.07 months in liquid-only groups.
Conclusions: The study highlights the potential value of integrating both biopsy modalities in selected clinical contexts.
A related commentary was published in the January 1 issue. The results of this trial were initially presented at the AACR Annual Meeting 2025.
Journal: Clinical Cancer Research (January 15 issue)
Purpose: This phase Ib/II trial (NCT04276493) assessed the antitumor activity, safety, and pharmacokinetics (PK) of zanidatamab in combination with tislelizumab and chemotherapy in patients with advanced HER2-positive (HER2+) gastric cancer/gastroesophageal junction cancer (GEJC).
Patients and Methods: Adult patients with previously untreated, unresectable, locally advanced/metastatic HER2+ gastric cancer/GEJC received zanidatamab 30 mg/kg i.v. (cohort A) or zanidatamab 1800 mg i.v. (weight <70 kg)/2,400 mg i.v. (weight ≥70 kg; cohort B) once every 3 weeks (Q3W). Both cohorts received tislelizumab 200 mg i.v. once every 3 weeks and standard chemotherapy [capecitabine and oxaliplatin (CAPOX)] once every 3 weeks. Primary endpoints were investigator-assessed confirmed objective response rate (cORR) per RECIST v1.1, in addition to the frequency and severity of adverse events (AE) and serious AEs. Secondary endpoints included investigator-assessed progression-free survival (PFS), duration of response (DoR), overall survival (OS), PK, and immunogenicity of zanidatamab.
Results: As of December 7, 2023, 33 patients (cohort A, n = 19; cohort B, n = 14) received treatment. The confirmed objective response rate was 75.8%; the median duration of response, progression-free survival, and overall survival were 23.3, 16.7, and 32.4 months, respectively. The most common treatment-related AEs (TRAEs) were diarrhea (100%), nausea (63.6%), and decreased appetite (48.5%). Treatment-related AEs of grade ≥3 were reported in 22 (66.7%) patients; diarrhea was the most common (27.3%).
Conclusions: Zanidatamab, in combination with tislelizumab and CAPOX, demonstrated clinically meaningful antitumor activity with a manageable safety profile as first-line therapy for patients with HER2+ gastric cancer/GEJC. These results support a further development of zanidatamab and tislelizumab with chemotherapy in this patient population in the ongoing phase III HERIZON-GEA-01 trial (NCT05152147).
Journal: Molecular Cancer Research
Genes encoding ETS family transcription factors are altered by chromosomal rearrangement in 60% to 70% of prostate cancers and nearly all Ewing sarcomas. Ewing sarcoma rearrangements result in chimeric fusion of ETS proteins to the RNA-binding protein EWSR1. Prostate cancer rearrangements result in aberrant expression of ETS proteins such as ETV1, ETV4, ETV5, or ERG that can interact with wild-type EWSR1, suggesting common mechanisms between these diseases. In this study, we find that ETV1, ETV4, and ETV5 can phenocopy EWSR1::FLI1 in Ewing sarcoma cell lines. However, rescue of EWSR1::FLI1 knockdown by ERG requires an ERG mutant that disrupts interaction with polycomb repressive complex 2 (PRC2). This suggests that EWSR1::ERG fusions that drive Ewing sarcoma avoid PRC2 interactions. We then identify an endogenous PRC2/FOXO1 complex and demonstrate that FOXO1 bridges ERG/PRC2 interaction. AKT-mediated degradation of FOXO1 and subsequent loss of the ERG/PRC2 interaction provide a mechanism for ERG synergy with PTEN deletion in prostate cancer.
Implications: These findings indicate that ETS transcription factors that drive prostate cancer and Ewing sarcoma utilize similar mechanisms and thus could be targeted by similar therapeutic approaches.
This article was highlighted in the January issue.
Journal: Molecular Cancer Therapeutics
DS-3939a: A TA-MUC1–Directed Antibody–Drug Conjugate with Broad Antitumor Activity
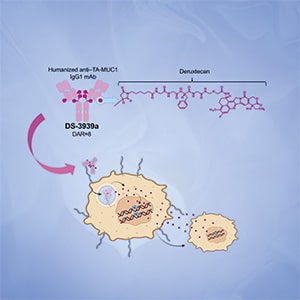
Tumor-associated mucin-1 (TA-MUC1) is a glycoform of the MUC1 protein that is aberrantly glycosylated and is primarily observed in cancer cells. TA-MUC1 is highly expressed in various human epithelial cancers, making it an attractive target for cancer therapies. In this study, we describe the development of DS-3939a, a novel TA-MUC1–targeting antibody–drug conjugate that utilizes the potent DNA topoisomerase I inhibitor DXd, and evaluation of its pharmacologic activity in preclinical in vitro and in vivo models. IHC of clinical tumor tissue microarrays of various cancer types exhibited positive staining for TA-MUC1 in a number of samples, with a particularly high positive rate in bladder, lung, and breast cancers. In vitro profiling of DS-3939a confirmed that it could specifically bind to TA-MUC1 and inhibit the growth of TA-MUC1–positive cancer cells by inducing DNA damage and apoptosis. DS-3939a also exhibited significant antitumor effects in multiple TA-MUC1–positive cell line–derived and patient-derived xenograft models. Moreover, DS-3939a elicited strong tumor regression in several xenograft models even following treatment with other cytotoxic antibody–drug conjugates, likely through its efficient payload delivery. Overall, these data provide evidence for the potential utility of DS-3939a for the treatment of TA-MUC1–expressing tumors and support the rationale for the ongoing phase I/II clinical study (NCT05875168).
This article was highlighted in the January issue where it was featured on the cover.
Journal: Cancer Research Communications
Cancer is a leading cause of death in Latin America and the Caribbean (LAC), and up-to-date estimates are essential to guide cancer policy. Using GLOBOCAN 2022 data, we analyzed cancer incidence and mortality across 32 LAC countries, calculated age-standardized rates, and assessed early-onset cancer (diagnosed at ages 15–50 years). Mortality-to-incidence ratios were used as a proxy for survival, joinpoint regression estimated annual percent change, and linear regression evaluated correlation between the Human Development Index and cancer indicators. In 2022, LAC recorded 1,551,060 new cancer cases (age-standardized incidence rate, 186.6 per 100,000) and 749,242 deaths (age-standardized mortality rate, 85.2 per 100,000). Prostate and breast cancers were the most common malignancies, whereas lung and breast cancers caused the highest mortality. Mortality declined for prostate cancer [annual percent change, −1.52; 95% confidence interval (CI), −1.80 to −1.24] and male lung cancer (−2.50; 95% CI, −2.68 to −2.33) but increased for female lung (+1.88; 95% CI, 1.71–2.05) and colorectal cancer (+2.48; 95% CI, 2.30–2.67). Human Development Index showed an inverse correlation with mortality-to-incidence ratio (P < 0.001), suggesting improved survival with higher development. Early-onset cancers represented 17% of new cases and 11% of deaths. These findings reveal a growing cancer burden and that persistent disparities in cancer epidemiology persist across LAC, highlighting the urgent need for targeted cancer control strategies and regional cancer control plans.
Significance: These findings highlight the urgent need for equity-focused cancer control policies, improved early detection, and expanded access to essential cancer care in the region.