Look Who’s Talking: How Electrical Communication Drives Melanoma
It sounds like something out of Frankenstein, but a new study in the AACR journal Cancer Discovery suggests that changes in electrical signals could spark cancer in certain skin cells.
Researchers found that the transfer of a neurotransmitter between two types of skin cells dampened electrical activity in the receiving cell and triggered the growth of melanoma, a deadly form of cancer that forms in pigment-producing skin cells known as melanocytes.
The findings unexpectedly implicate neurotransmitters and electrical activity—typically associated with neuronal communication—in cancer development, noted Mohita Tagore, PhD, a postdoctoral research scientist at Memorial Sloan Kettering Cancer Center (MSK) and the first author of the study.
Beyond uncovering a novel facet of melanoma development, the results also present potential opportunities for treating or preventing the aggressive skin cancer.
The observations by Tagore and senior author Richard White, MD, PhD, a physician-scientist at MSK and Ludwig Cancer Research at the University of Oxford, grew out of their curiosity about cell-cell communication and its potential impacts on cancer.
“The traditional view of cancer is that a mutation causes a cell to become cancerous, and the microenvironment conducive to its growth forms around the cancer,” said White.
But a different view of cancer, he noted, is that it is a community of cells from the onset.
“Which cells within this community are talking to each other in the earliest stages, even before cancer forms? Does that early communication somehow influence the ability to form a tumor?” he asked.
White and Tagore chose to explore this topic in melanoma, where mutations alone do not explain cancer development. While half of all melanomas harbor mutations in the BRAF oncogene, these mutations are present in benign skin moles as well.
“This suggests that BRAF mutation is not sufficient for melanoma development and raises the question of why certain BRAF-mutated melanocytes develop into cancer, while others remain benign,” said White. He and colleagues hypothesized that interactions within the community of skin cells could play a role, homing in on a particular interaction between melanocytes and surrounding skin cells known as keratinocytes.
He explained that melanocytes intrinsically communicate with keratinocytes through vesicles that carry various molecules, such as melanin, from one cell to the other. The transfer of melanin from melanocytes to keratinocytes is what gives skin its color, he added.
“Skin cells are doing this on a daily basis,” he said. “We wanted to know if cancer cells are hijacking this communication for their own benefit.”
To investigate if vesicle-mediated communication contributed to cancer, White and Tagore conducted a series of genetic experiments in zebrafish, a popular model system for melanoma due to the melanin-containing stripes that give the fish its name.
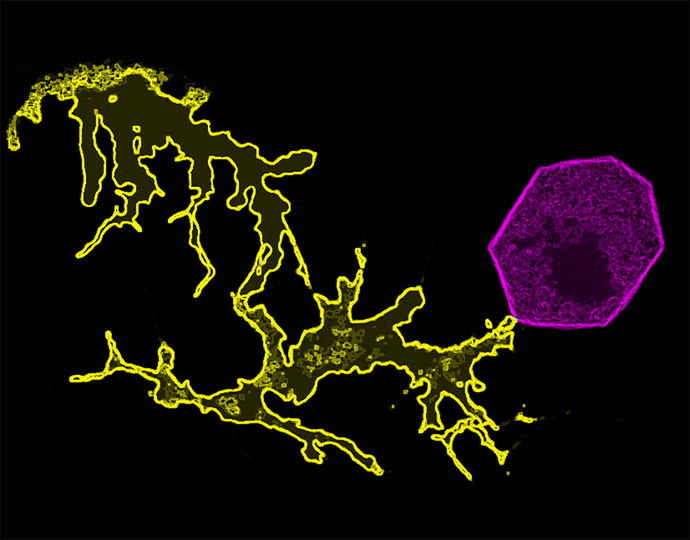
They engineered the zebrafish such that the transfer of vesicles from BRAF-mutated melanocytes to keratinocytes led to the expression of a fluorescent protein within the receiving (or “switched”) keratinocyte, turning the keratinocyte red. The transparent skin of this particular zebrafish strain allowed researchers to easily observe the change in color, which revealed that this mode of communication occurred almost exclusively between melanocytes and keratinocytes that were in direct contact with one another.
When the researchers selectively killed off the switched keratinocytes, they found that melanoma was less likely to develop, suggesting that communication between melanocytes and keratinocytes was required for melanoma initiation.
Similar results were observed when the experiments were repeated in human and mouse cell cultures.
Further investigation revealed that vesicle-mediated communication and melanoma development relied on the transfer of the neurotransmitter GABA from BRAF-mutated melanocytes to GABA-specific receptors on keratinocytes, a surprising finding given that GABA signaling is usually associated with neurons rather than skin cells, the researchers noted.
“I would not have necessarily expected a neurotransmitter to be involved in the communication between skin cells,” said White. “Interactions between neurons and brain cancer cells have been reported, but here we observed neuronal-like communication occurring between two non-neuron cells.”
In neuronal communication, GABA inhibits a neuron’s ability to send or receive electrical signals. The researchers observed a similar effect in skin cells, as GABA inhibited electrical activity in keratinocytes and induced keratinocytes to secrete LIF1, a protein known to promote melanoma.

Exposure to a GABA agonist led to increased vesicle transfer in their model, while a GABA antagonist suppressed vesicle transfer. Tagore noted that these findings raise the possibility of inhibiting GABA to treat melanoma or to prevent the progression of benign skin lesions to cancer; however, she cautioned that additional research is needed to understand the clinical implications of this work.
The researchers also found that melanoma cells had increased expression of genes involved in GABA production compared with nonmalignant melanocytes, highlighting a difference that may underlie the transition to cancer in certain melanocytes.
Together, the results suggest that some BRAF-mutated melanocytes may upregulate and transmit GABA to promote vesicle-mediated communication between melanocytes and keratinocytes, dampen the electrical activity of neighboring keratinocytes, and initiate the progression to melanoma, Tagore summarized.
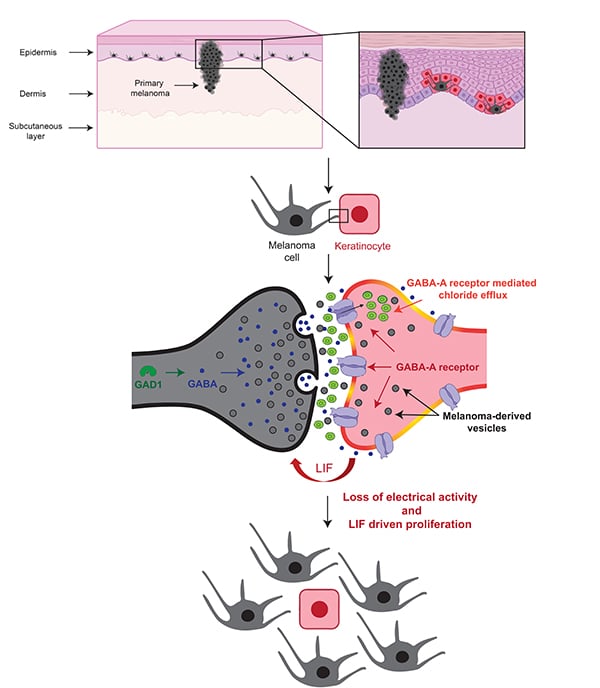
“Something about normal electrical activity in keratinocytes appears to suppress the progression of BRAF-mutated melanocytes to melanoma,” she said. “Our findings indicate that some BRAF-mutated melanocytes are able to modulate this electrical activity through GABA in order to progress to melanoma.”
Tagore is now exploring interactions between melanoma cells and neighboring immune cells to determine if a similar type of electrical signaling could contribute to immune suppression in the tumor microenvironment.
While the study was performed in melanoma models, White anticipates that similar modes of communication may exist in other cancer types as well. “Different cancers may employ different neurotransmitters, but I think the general mechanism is likely relevant across cancers,” he said.
“Electrical signaling has probably existed since the very earliest stages of cell evolution, so I find it fascinating that cancers may be usurping this evolutionarily ancient form of communication to promote their own growth,” he added.
The study builds on prior work from White’s lab on melanoma development and progression, research that has been largely driven by curiosity about how cancers manipulate basic cellular processes.
Looking forward, White is interested in understanding how electrical communication might impact cell fate. He is also curious about how the macroenvironment—which encompasses environmental exposures, medications, diet, and other external factors—impacts interactions within the tumor microenvironment.
“I’ve always been amazed by the power of just being curious about something,” said White. “What started as intrigue about a basic function of skin cells is giving us new insights into how cancer forms and how we might be able to treat it.”

For her part, Tagore is grateful for having had the opportunity to follow her scientific curiosity on this project.
“I had the privilege of wandering in the dark, of taking my time to figure this out,” she said. “For me as a trainee, that was incredibly rewarding and an immense learning experience.”
As a researcher, she is driven by a quote she first heard as a graduate student—a quote that has ended up aligning remarkably well with her postdoctoral research in White’s lab:
The cell is always talking. The secret is to understand its language.
She summed it up this way: “Regardless of what you’re studying—whether it’s cancer, autoimmune disease, infectious disease—we are all in some way trying to understand what our cells are telling us.
“If you’re driven by curiosity, driven to understand how cells are communicating and what they’re saying, eventually you’re going to make progress against a lot of different diseases.”