
FDA Approves New PARP Inhibitor for BRCA-mutant Ovarian Cancer
On Monday, the U.S. Food and Drug Administration (FDA) announced good news for a subgroup of patients with ovarian...


On Monday, the U.S. Food and Drug Administration (FDA) announced good news for a subgroup of patients with ovarian...

On Tuesday, President Barack Obama signed H.R. 34, the 21st Century Cures Act (Cures), into law. The expansive bill...
Lesbian, gay, bisexual, and transgender (LGBT) people may be at increased risk of certain cancers.

The FDA has approved nivolumab for treating patients with recurrent or metastatic squamous cell carcinoma of the head...

The AACR is a founder and organizing partner of the Rally for Medical Research, which took place for the...

This month is Breast Cancer Awareness Month, a type of cancer against which we have made much progress. However,...

Last week, the U.S. Food and Drug Administration (FDA) added a new therapeutic to the armamentarium for oncologists treating...

Earlier this week, the U.S. Food and Drug Association (FDA) announced it had approved the immunotherapeutic atezolizumab (Tecentriq) for...
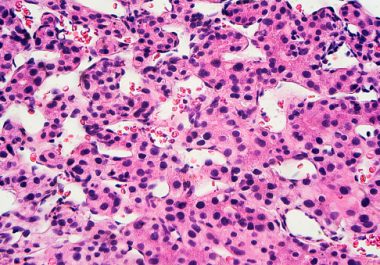
Liver cancer rates have been on the rise in the United States and have tripled in the last four...
The potential for developing new immunotherapies and expanding the use of existing ones will be the focus of the...