
AACR Virtual Annual Meeting II: Current Events Meet Cancer Science
On April 27, cancer researchers, health care professionals, patients, advocates, and policymakers around the world logged on to their...


On April 27, cancer researchers, health care professionals, patients, advocates, and policymakers around the world logged on to their...

By Carmine S. Leggett, PhD Clinical trials in oncology have changed the face of cancer treatment, allowing cancer researchers...
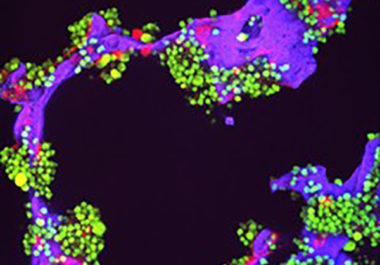
By Trevan Locke, PhD Thanks to a wave of newly approved therapies in the past two decades, survival rates...
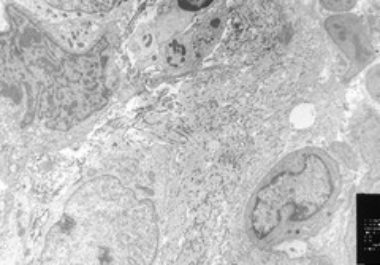
The first new anticancer therapeutics approved by the U.S. Food and Drug Administration (FDA) in 2020 are for the...

The U.S. Food and Drug Administration (FDA) approved a new molecularly targeted therapeutic called fam-trastuzumab deruxtecan-nxki (Enhertu) for treating...
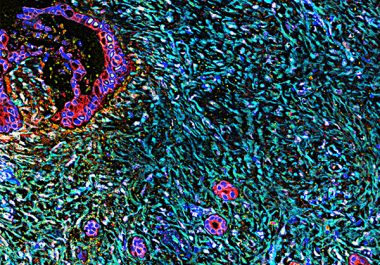
In the final days of 2019, the U.S. Food and Drug Administration (FDA) expanded the use of a previously...

In 2019, research continued to drive progress across the spectrum of cancer care in the form of new and...

In November and the first half of December, the U.S. Food and Drug Administration (FDA) announced three approvals for...

As 2019 draws to a close, the American Association for Cancer Research (AACR) is grateful for our growing ranks...

Earlier this month, the U.S. Food and Drug Administration (FDA) announced that the Office of Hematology and Oncology Products...