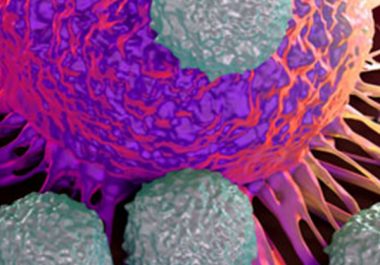
A New Immunotherapy for Multiple Myeloma
Elotuzumab became the third new treatment for multiple myeloma approved by the U.S. Food and Drug Administration in a...


Elotuzumab became the third new treatment for multiple myeloma approved by the U.S. Food and Drug Administration in a...

Daratumumab is the first monoclonal antibody approved by the U.S. Food and Drug Administration for patients with multiple myeloma...

The U.S. Food and Drug Administration approval provides a new option for patients whose disease is driven by mutations...
The U.S. Food and Drug Administration approval provides a new option for patients with the most deadly form of...
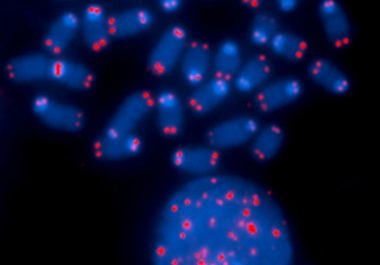
The U.S. Food and Drug Administration approval provides a new option for patients with liposarcoma and leiomyosarcoma that is...
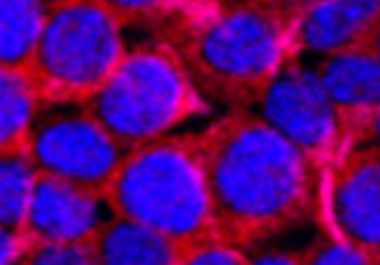
The U.S. Food and Drug Administration approval provides a new option for patients who face a poor prognosis Onivyde,...
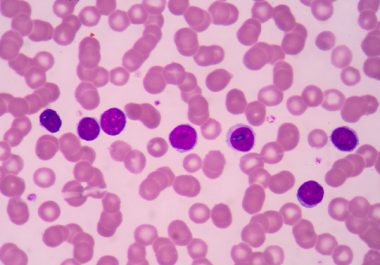
The U.S. Food and Drug Administration approval provides a new option for patients whose disease has worsened after treatment...

The FDA-approved therapeutic is the first to target the T790M EGFR mutation that frequently causes resistance to other EGFR-targeted...
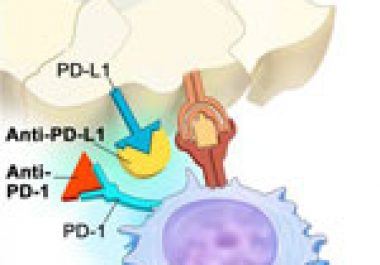
The U.S. Food and Drug Administration approval expands the use of the immunotherapeutic pembrolizumab to certain patients with the...

Approved immunotherapeutics release different brakes on the immune system. A new combination of cancer immunotherapeutics – ipilimumab (Yervoy) and...