A New HPV Vaccine May Prevent More Cervical Cancers Than Existing Vaccines
A recent study found that an investigational HPV vaccine could prevent nearly 90 percent of cervical cancers.
Human papillomavirus (HPV) is the major cause of cervical cancer, an often preventable cancer diagnosed in more than 12,000 women a year in the U.S., according to federal statistics. More than 4,000 women a year die of cervical cancer in this country.
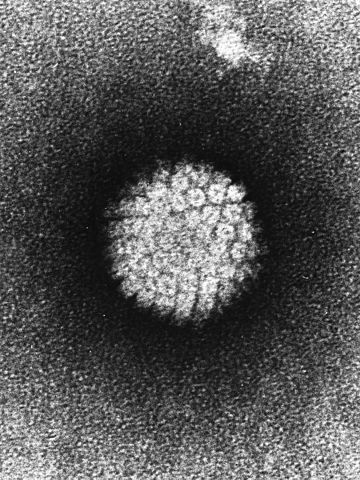
But many of these cancer cases and deaths could be avoided. Two U.S. Food and Drug Administration-approved vaccines, Gardasil and Cervarix, can prevent infections with two subtypes of the virus that cause the majority of cervical cancers. And an investigational HPV vaccine that provides protection against nine subtypes of the virus – a nine-valent vaccine – is currently under review by the FDA.
In a study published in Cancer Epidemiology, Biomarkers & Prevention, a journal of the American Association for Cancer Research (AACR), researchers investigated the types of HPV infections in 12,514 women, ages 15 to 45, enrolled in the placebo arms of three clinical trials testing the nine-valent, investigational HPV vaccine.
They found that about 91 percent of the most advanced cervical precancers had seven of the nine HPV subtypes covered by the investigational nine-valent vaccine. Therefore, nine in 10 cases of cervical cancers can be prevented by use of the new HPV vaccine.
“If vaccination programs with this new-generation vaccine are effectively implemented, approximately 90 percent of invasive cervical cancer cases worldwide could be prevented, in addition to the majority of precancerous lesions,” said senior author Elmar A. Joura, MD, an associate professor of gynecology at the
Medical University of Vienna in Austria.
Besides cervical cancer, HPV is also associated with several other cancer types, including vulvar, vaginal, penile, anal, as well as oral and other head and neck cancers.
“It can be difficult to discuss getting a sexually transmitted cancer, but it is time to talk about it,” says Bob Margolis, a sports writer and a father of three, who beat cancer three times including HPV-related head and neck cancer. “We can prevent a generation of young Americans from having to go through the same experience I did.”
Margolis shares his experience with a diagnosis of HPV-related stage IV head and neck cancer, and his commitment to encouraging people to talk about it and get help, in this video:
“Despite the clear safety profile of the currently disseminated HPV vaccines, uptake in the United States and other resource-rich countries has been inadequate,” said Dr. Joura. “To achieve the population-level potential of the HPV vaccine to reduce cancer, vaccine uptake must increase.”
There is a lack of public awareness and adherence to vaccination programs in the U.S.
The Centers for Disease Control and Prevention (CDC) recommends vaccination for girls and boys ages 13 to 17. But in 2013, only 57.3 percent of girls in this age group had received at least one dose of HPV vaccine. Only one in three girls received the recommended three doses. In some states, that number is as low as 12 percent. Only 34.6 percent of teenage boys received vaccination in 2013.
