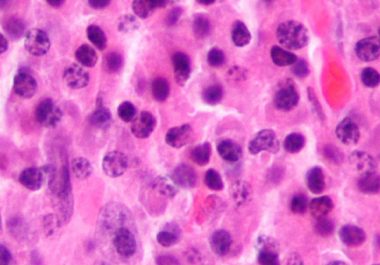
Preventing Bone Complications for Multiple Myeloma Patients
The FDA expanded the use of a molecularly targeted therapeutic to include the prevention of bone complications in patients with multiple myeloma.


The FDA expanded the use of a molecularly targeted therapeutic to include the prevention of bone complications in patients with multiple myeloma.
The FDA approved a molecularly targeted therapeutic for treating certain patients who have breast cancer that tests positive for a cancer-associated BRCA1 or BRCA2 mutation.

An American Association for Cancer Research (AACR) Special Conference highlighted the connections between weight and cancer.
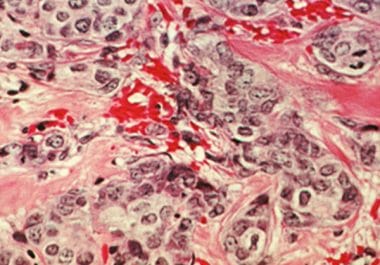
Whole-genome sequencing may help identify the patients who are most likely to benefit from certain types of chemotherapy.

Thyroid cancer survivors – especially those diagnosed before age 40 – have elevated risk for several aging-related diseases.
A Q&A with medical oncologist Patricia Ganz on chemotherapy-related nerve damage.

More Americans are beating childhood cancer, but many experience effects later in life such as high blood pressure.

Study: Urban native populations may have decreased survival following invasive prostate and breast cancer. Urban American Indians and Alaskan Natives (AIAN) appear to have lower survival rates following invasive prostate and breast cancer as...

Study: Non-obese diabetic African-American women may have a higher risk of developing ER-negative breast cancer.

The type of fat and its location in the body appear to play a role in the risk of developing cancer, a study in the AACR journal Cancer Prevention Research.