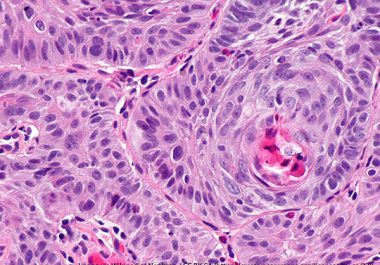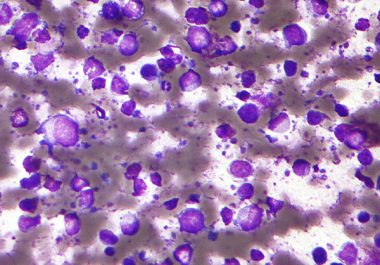
Treating an Aggressive Type of Non-Hodgkin Lymphoma
The FDA has approved a new immunotherapeutic for treating certain adult patients with diffuse large B-cell lymphoma. The U.S. Food and Drug Administration (FDA) granted accelerated approval to a new targeted immunotherapeutic called tafasitamab-cxix...