Remodeling of the Tumor Microenvironment in Ewing Sarcoma

Ewing sarcoma (EwS) is a mesenchymal-derived cancer of the bone and soft tissue primarily occurring in children and adolescents. The EWS::FLI1 fusion protein is a common initiator of EwS tumors. However, rare sub-populations of EwS tumor cells have been found that express low levels of EWS::FLI1 and increased expression of mesenchymal lineage genes that are normally repressed by the fusion protein (1,2). A recent study published in Clinical Cancer Research (3) uncovered a mechanism by which these EWS::FLI1-low, mesenchymal-high state tumor cells remodel the tumor microenvironment, by depositing extracellular matrix (ECM) proteins in EwS tumors.
The study was co-led by Emma Wrenn, PhD, and April Apfelbaum, PhD. A postdoctoral fellow at Seattle Children’s Research Institute, Dr. Wrenn received the AACR-QuadW Foundation Sarcoma Research Fellowship, in memory of Willie Tichenor in 2022 for her research project titled “Targeting mesenchymal cell states in Ewing Sarcoma.”
Dr. Wrenn and her colleagues first identified CD73 expression as a potential marker of cells that have lost EWS::FLI1-mediated repression of mesenchymal development genes and low EWS::FLI1 activity. They confirmed that CD73+ EwS cells displayed mesenchymal morphologies, generated extensive F-actin rich membrane protrusions, and migrated faster than CD73– cells. However when the researchers measured the level of EWS::FLI1, they unexpectedly found no discernable difference in the expression of EWS::FLI1 transcript or protein between CD73+ and CD73– cells. Further, they found that although CD73+ cells expressed high levels of the EWS::FLI1-repressed gene signature, this was not accompanied by a loss of EWS::FLI1-dependent gene activation or expression of the cell cycle program positively regulated by the fusion protein.
To further investigate the dissociation between EWS::FLI1-activated and -repressed signatures in CD73+ cells, the research team performed single-cell proteogenomic profiling using CITE-Seq and found profound heterogeneity among the transcriptomes and cell surface CD73 expression across 9 EwS cell line models. By comparing CD73+ and CD73– cells they found that across all models, CD73+ cells showed upregulation of the epithelial mesenchymal transition (EMT) signature and ECM genes normally repressed by EWS::FLI1.
The researchers then analyzed tumor xenografts and confirmed that the transcriptional and spatial heterogeneity of CD73+ cell subpopulations observed in vitro was also present in in vivo models of EwS. Also reflective of the in vitro findings, they found that EwS tumor cells that highly expressed the ECM gene signature showed loss of EWS::FLI1-dependent gene repression. However loss of this repressive signature was not always accompanied by loss of EWS::FLI1-mediated gene activation, uncovering hybrid transcriptional states.
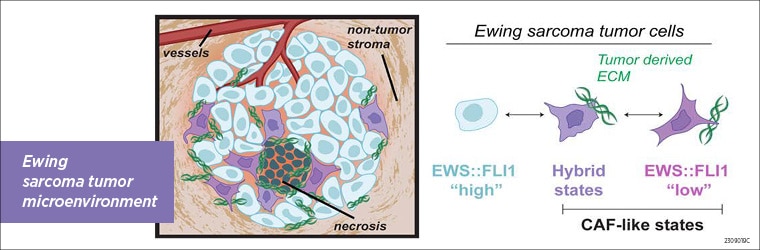
Explaining the significance of these research findings, Dr. Wrenn said, “Cancer associated fibroblasts (CAFs) play a critical role in many tumor types by depositing ECM proteins which promote disease progression and treatment resistance. Here we find that Ewing sarcomas, which do not typically contain many CAFs, in fact generate subpopulations of tumor cells that themselves remodel the ECM in a “CAF-like” manner. This raises the possibility of therapeutically targeting these cells and their functions in tumor microenvironment remodeling.”
She went on to say, “Support from this AACR/QuadW foundation fellowship has been so helpful for jump-starting this project during the early phase of my postdoctoral career. And sharing this work with the rest of the AACR community at the annual meeting led to some very helpful conversations and future directions that have guided our efforts to dig deeper into the molecular mechanisms underlying these findings.”
References:
- Franzetti GA, Laud-Duval K, van der Ent W, Brisac A, Irondelle M, Aubert S, et al. Cell to-cell heterogeneity of EWSR1-FLI1 activity determines proliferation/migration choices in Ewing sarcoma cells. Oncogene. 2017;36(25):3505-14.
- Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, and Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer cell. 2007;11(5):421-9.
- Wrenn ED, Apfelbaum AA, Rudzinski ER, Deng X, Jiang W, Sud S, Van Noord RA, Newman EA, Garcia NM, Miyaki A, Hoglund VJ, Bhise SS, Kanaan SB, Waltner OG, Furlan SN, Lawlor ER. Cancer-associated fibroblast-like tumor cells remodel the Ewing sarcoma tumor microenvironment. Clin Cancer Res. 2023 Jul 20:CCR-23-1111. doi: 10.1158/1078-0432.CCR-23-1111. Epub ahead of print.