New Molecularly Targeted Therapeutic Approved by FDA for Numerous Blood Disorders
At the end of last week, the U.S. Food and Drug Administration (FDA) announced the approval of the first molecularly targeted therapeutic for acute myeloid leukemia (AML), midostaurin (Rydapt). They also approved a companion diagnostic test to identify those AML patients eligible to receive it: adults newly diagnosed with AML harboring a mutation in the FLT3 gene.
With these decisions, midostaurin became the first therapeutic approved specifically for treating patients with FLT3 mutation–positive cancer.
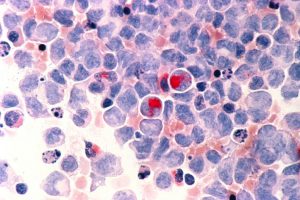
Human cells with acute myelocytic leukemia (AML) in the pericardial fluid. Image courtesy of the National Cancer Institute.
Midostaurin is intended for use in both the induction phase (chemotherapy given to induce remission) and consolidation phase (chemotherapy given to sustain a remission) of AML treatment, in combination with standard cytarabine and daunorubicin induction chemotherapy and cytarabine consolidation chemotherapy.
Alongside these decisions, the FDA also approved midostaurin as a monotherapy for treating adults with certain aggressive forms of a rare disorder known as systemic mastocytosis—aggressive systemic mastocytosis, systemic mastocytosis with associated hematological neoplasm, and mast cell leukemia.
Why approve midostaurin for AML?
AML is the form of leukemia most commonly diagnosed in the United States; in 2017, it is anticipated that there will be 21,380 new cases of the disease. With an overall five-year relative survival rate of just 27 percent, it is also the form of leukemia that carries the worst prognosis.
About 25 percent of AML cases are characterized by the presence of mutations in the FLT3 gene. Patients with this form of AML have particularly poor outcomes.
Because the FLT3 mutations lead to constitutive FLT3 activation, which promotes survival and proliferation of the leukemia cells, researchers set out to investigate whether targeting FLT3 might provide a new approach to treating patients with FLT3 mutation–positive AML.
Midostaurin targets several related molecules called tyrosine kinase receptors, including FLT3 and KIT. According to the FDA statement, in the phase III clinical trial that led to the approval, patients with FLT3 mutation–positive AML were randomly assigned to receive either midostaurin or placebo during both the induction and consolidation phases of standard treatment.
The results of the trial, which were presented at a conference in December 2015, showed that patients who received midostaurin plus standard induction and consolidation chemotherapy had a 23 percent improvement in overall survival compared with those patients who received placebo.
Why approve it for systemic mastocytosis?
Mastocytosis is a rare disorder that occurs when immune cells called mast cells accumulate in skin and/or internal organs such as the liver, spleen, bone marrow, and small intestines. Until last year, it was considered a subtype of myeloproliferative neoplasms, but it is now classified as a separate disease category. There are two main forms of the disorder, cutaneous mastocytosis and systemic mastocytosis. According to the National Institutes of Health, the exact incidence and prevalence of mastocytosis are not known.
Systemic mastocytosis encompasses the forms of mastocytosis that affect more than one part of the body. Most cases are caused by mutations in the KIT gene that lead to constitutive KIT activation, which promotes aberrant survival and proliferation of the mast cells.
Given that KIT is one of the tyrosine kinases targeted by midostaurin, researchers tested it as a treatment for the three most aggressive forms of systemic mastocytosis—aggressive systemic mastocytosis, systemic mastocytosis with associated hematological neoplasm, and mast cell leukemia.
According to the FDA statement, in the phase II clinical trial that led to the approval of midostaurin for these disorders, one patient with mast cell leukemia had a complete remission. The rates of confirmed complete remission plus incomplete remission, as assessed by modified Valent criteria, were 38 percent for aggressive systemic mastocytosis and 16 percent for systemic mastocytosis with associated hematological neoplasm.



