AACR Annual Meeting 2023: Therapy Advances for Patients With Rare Melanoma Types
The treatment of cutaneous melanoma has been profoundly transformed by the advent of targeted therapy and immunotherapy, which have prolonged survival in some patients with metastatic disease and reduced the risk of relapse in some with early-stage disease.
Advances in treatment of other rare types of melanoma have lagged behind. However, the past year has seen significant progress, when the U.S. Food and Drug Administration approved the new therapeutic tebentafusp (Kimmtrak) for uveal melanoma. In addition, advances in biomarkers of response to tebentafusp and informative clinical data on immunotherapy for desmoplastic melanoma were presented at the AACR Annual Meeting 2023.
A New Type of Immunotherapy Extends the Life of Patients With Uveal Melanoma
Uveal melanoma, or melanoma of the eye, accounts for 5% of all melanomas, with an incidence of 4-5 cases per million individuals in the U.S. It develops in a part of the eye called the uvea, in pigment-producing cells called melanocytes. When diagnosed early, uveal melanoma is treatable with surgery or radiation, but can be fatal when it metastasizes, which happens in half of the cases.
Even though uveal melanoma has the same cell of origin as cutaneous melanoma, it has distinct molecular drivers and tumor microenvironment features, and the systemic therapies that have been successful for cutaneous melanoma have not shown benefit for uveal melanoma. For example, uveal melanoma does not display the high mutational burden that characterizes cutaneous melanoma, and this may contribute to the low clinical response to immune checkpoint inhibitor therapy. Until recently, there were no approved therapies for uveal melanoma.
That changed in 2022, with the approval of tebentafusp, the first molecule of a new class of therapeutics called immune-mobilizing monoclonal T-cell receptors against cancer (ImmTACs), for certain adult patients with unresectable or metastatic uveal melanoma.
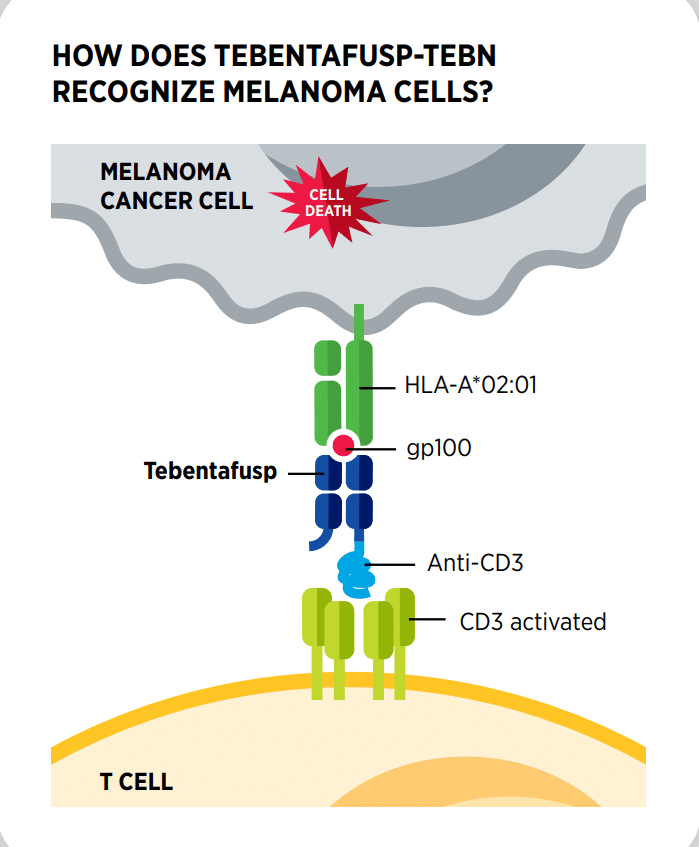
Tebentafusp directs and activates the cancer-killing ability of T cells against melanoma. It is a bispecific protein that functions as a bridge between cancer cells and T cells by simultaneously binding the glycoprotein 100 (Gp-100) antigen, which is highly expressed on the surface of melanoma cells, and CD3, a molecule involved in the activation of T cells. Tebentafusp is only approved for patients whose cells have a specific human leukocyte antigen (HLA) on their surface known as HLA-A*02:01. HLA proteins are involved in the activation of the immune response.
The approval of tebentafusp was supported by the results of a clinical trial presented at the AACR Annual Meeting 2021. In this study, patients with uveal melanoma treated with tebentafusp had their risk of death nearly halved compared with patients assigned to the control group, who were treated with immune checkpoint inhibitors or chemotherapy.
A recent analysis of circulating tumor DNA (ctDNA) presented at the AACR Annual Meeting 2023 showed that, before starting treatment, most of the patients on this trial had detectable circulating mutations in one or more genes that are commonly altered in uveal melanoma. After nine weeks of treatment, the researchers found a drop in mutant ctDNA in nearly 90% of these patients, with bigger reductions correlating with longer overall survival, even in patients who experienced increased tumor burden. These findings suggested that ctDNA reduction occurring early in the course of treatment may be a better indication of tebentafusp efficacy in this patient population than tumor response based on imaging tests.
Fine-tuning Immune Checkpoint Inhibitor Therapy for Patients With Desmoplastic Melanoma
Desmoplastic melanoma is another rare subtype of melanoma that typically affects parts of the body that are chronically exposed to the sun, including the head and neck. It is characterized by an abundant stromal component and is often not pigmented, making the diagnosis challenging.
The standard treatment consists of surgery, in some cases followed by radiation, but the rates of local recurrence are high. Advanced or metastatic desmoplastic melanoma is treated with the same therapies used for the more common subtypes, including immune checkpoint inhibitors, which are commonly given in combination.
Recently, a clinical trial was conducted to assess the efficacy of the checkpoint inhibitor pembrolizumab specifically in patients with desmoplastic melanoma, both resectable and metastatic.
In one cohort, pembrolizumab given as a neoadjuvant therapy before surgical resection resulted in a high pathologic complete response rate (59%), meaning that when patients underwent surgery, in more than half of them there was no cancer tissue remaining. This is particularly impactful because resection of desmoplastic melanoma tumors typically requires large excisions that result in disfiguring scars.
Another part of the trial assessed pembrolizumab treatment in patients with metastatic disease. Results from this cohort, presented at the AACR Annual Meeting 2023, demonstrated an exceptionally high complete response rate (33%) and overall response rate (89%), exceeding those observed in previous trials of immune checkpoint blockade in other melanoma subtypes.

“The high responses to pembrolizumab monotherapy indicate that first-line combination therapy may not be necessary for patients with desmoplastic melanoma, which could help patients avoid unnecessary toxicities,” said lead author Kari Kendra, MD, PhD, professor of medicine and director of cutaneous oncology at The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, in an AACR press release.
The researchers believe that the high mutational burden may partially explain the exceptionally high response to immune checkpoint inhibitor therapy observed in these patients.
Based on this trial, single-agent pembrolizumab may become the treatment of choice for patients with inoperable desmoplastic melanoma, as explained in detail in a News in Brief feature in Cancer Discovery. This trial exemplifies how studying rare cancers can help identify the best therapeutic approach for each patient.