Experts Forecast 2024, Part 3: Precision Medicine for Cancer Treatment
In anticipation of the new year, we asked four experts to predict the advances we might see in 2024 in their respective fields. In part 3 of this four-part series, we share thoughts from AACR President-Elect Patricia M. LoRusso, DO, PhD (hc), FAACR, on advances in precision medicine.
Check out the previous installments of the series:
- Part 1 featured predictions from Catherine J. Wu, MD, FAACR, for cancer vaccines
- Part 2 featured predictions from Robert A. Winn, MD, FAACR, for achieving health equity
In recent decades, almost half of all newly approved cancer therapies have been precision medicine—that is, therapies designed to home in on tumors by targeting the molecular alterations that set them apart from normal tissues.

The growth of precision medicine has been spurred, in large part, by technological advances, according to AACR President-Elect Patricia M. LoRusso, DO, PhD (hc), FAACR, a professor of medicine, chief of the early phase clinical trials program, and associate cancer center director for experimental therapeutics at the Yale Cancer Center and Smilow Cancer Hospital.
In 2024, LoRusso expects that technological advances will continue to drive progress in the precision medicine field, enabling researchers and clinicians to drug more targets, use precision medicine earlier in treatment, and personalize combination therapies.
“Every year, there are new technological advances that are allowing us to identify novel targets, design drugs against these targets, and define the mechanisms of drug response and treatment resistance,” she said. “The knowledge gained in the biology of cancer has led to more focused, target-specific agents.”
Drugging the undruggable
One area in which LoRusso expects advances is developing drugs against targets that have long been considered “undruggable.”
“We are seeing significant advancement in developing novel drug therapies that target molecular alterations we were not able to effectively target before,” she said, citing the development and approval of inhibitors against KRAS G12C mutations as evidence of recent progress.
“I think this year is going to bring exciting data on novel drug combinations that are both tolerable and effective in tumors with so-called ‘undruggable’ alterations, such as p53 Y220C, KRAS G12D, and potentially other KRAS molecular alterations as examples,” LoRusso added. “I anticipate we will have more of these drugs entering the clinic this year, which will hopefully be a game-changer for patients.”
Improving antibody-drug conjugates
She also predicts that advances in antibody-drug conjugates (ADCs) will allow researchers to target a wider range of alterations.
ADCs are a type of precision medicine therapy in which a toxic drug is tethered to a monoclonal antibody; when the antibody binds to a target protein on the cancer cell or even with some ADCs in the tumor microenvironment (TME), the ADC is internalized, and the toxic drug is released into the cell (or TME) where it causes cell death.
The collection of compounds that comprise an ADC—a monoclonal antibody, a toxic payload, and a chemical linker that connects the two—presents multiple opportunities for optimization, LoRusso noted. “This year, I think we are going to build our understanding about how all of these components impact tumor biology and how they can be fine-tuned to more effectively attack cancer.”
In 2024, she anticipates greater development of ADCs that incorporate bispecific antibodies, which can potentially allow them to pinpoint novel targets and increase tumor specificity. Further, she expects researchers to explore unique payloads and improved chemical linkers that promote drug stability and minimize systemic toxicity.
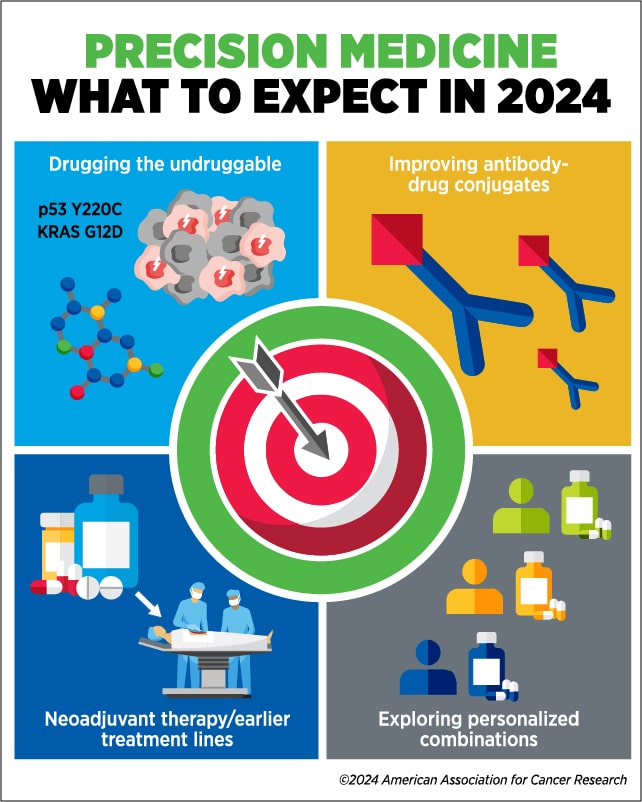
Using precision medicine earlier
LoRusso anticipates greater interest in 2024 in using precision medicine for earlier stages of disease and in earlier lines of cancer treatment, including as neoadjuvant treatment.
“If you can target a tumor’s specific alterations sooner, the potential exists for a greater possibility of eradicating the tumor,” she explained. “At earlier stages or lines of treatment, the tumor is less likely to have spread or developed additional molecular alterations that can make it harder to treat with precision medicine.”
This year, LoRusso expects to see more biomarker-driven trials testing whether therapies that have historically been used as adjuvant treatment for metastatic disease could be effective in the neoadjuvant setting. She also anticipates that newly developed ADCs or small-molecule inhibitors will increasingly be used as neoadjuvant therapy.
“If we can define appropriate tumor drivers and target them as early as possible, we might be able to think about curing cancer rather than simply controlling it,” she said.
Personalizing combination therapies
Combining therapies is a tried-and-true strategy in cancer treatment, but LoRusso argued that the impersonal approach currently used is hindering progress.
“One thing that is missing as we are designing combinations is thinking about the ratios of the individual drugs that comprise the combination. How do we know that we need full doses of both drugs? What if we need a little bit more of one drug and less of another?” she posed, explaining that some molecular alterations in any given tumor may be more relevant than others in driving tumor progression.
“Genomic tumor profiling tells us which alterations exist, but it doesn’t tell us how functional they are,” she said, noting that some of the alterations identified in a tumor may no longer be relevant to the disease. Emerging technologies could help clinicians understand the functionality of each alteration and quantify its significance relative to other alterations. Phosphoproteomic profiling, for example, can identify which proteins in the cell are phosphorylated, a common mark of protein activation.
LoRusso hopes that in 2024 and beyond, researchers will consider using this information to develop and evaluate personalized combinations unique to each tumor’s “ratio of alterations.”
“I dream of the day when we can use phosphoproteomics to not only better understand which alterations we should be targeting based on their functionality, but also potentially use phosphoproteomics as a road map to define the optimal ratio of each drug within a combination,” she said.
“The emergence of increasingly sophisticated technology has led to novel drugs, novel trial designs, novel combination strategies, novel technologies for biomarker development and assessment—all driven by a greater understanding of cancer biology,” LoRusso concluded.
“These are very, very exciting times for precision medicine and cancer drug development.”