Editors’ Picks, November 2025: Sucralose’s Effect on Immunotherapy, Benefits of Caloric Restriction on Prostate Cancer Treatment, and More
As November draws to a close, many are preparing to gather for a celebration of everything they are thankful for. Others are anticipating the following day’s slew of Black Friday deals that are too good to pass up. Meanwhile, the editors of the 10 peer-reviewed journals published by the American Association for Cancer Research (AACR) are combining the best of both worlds with a hearty helping of exciting studies you can’t help but be thankful for at a deal you absolutely can’t pass up—free to read at the links below for a limited time.
But before you dig into these abstracts, let us first whet your appetite. One group of researchers found that nonnutritive sweeteners like sucralose could make immunotherapy less effective. Another examined whether the ingestion of butyrylated high-amylose maize starch could help to reduce the risk of colorectal cancer. And diet was also top of mind for researchers looking into the effect of caloric restriction on the efficacy of antiandrogen therapy for prostate cancer.
Of course, not all the studies are related to what you eat. You can also learn about a gene signature that may help predict which diffuse large B-cell lymphoma cells will become resistant to CAR T-cell therapy, a couple of early-phase clinical trials of dual-targeting therapeutics, how neighborhood disadvantage can lead to the epigenetic reprogramming of key oncogenesis pathways, and much more.
Journal: Blood Cancer Discovery
Current understanding of lymphoma cell-intrinsic mechanisms of relapse following chimeric antigen receptor (CAR) T-cell treatment of diffuse large B-cell lymphoma (DLBCL) include antigen loss and apoptosis resistance. Herein, CD19 CAR T-cell response and resistance were modeled, and it was identified that treatment-naïve CD19 expression does not correlate with CAR T-cell sensitivity, but resistance is frequently accompanied by reversible downregulation of CD19 that once restored is not paralleled with restored sensitivity to CAR T cell–mediated killing. Profiling a suite of DLBCL cell lines to CD19 CAR T-cell sensitivity reveals that DLBCL cells become nonresponsive to CAR T cell–killing, including to alternative antigen targeting of CD20 or CD22. Leveraging these resistant models, we identified gene signatures present in the CAR T cell–resistant DLBCL cell lines that correlate with patient response to CTL019 in two independent clinical trials. Finally, we show that combination strategies to overcome this resistance, including up-front dual-antigen targeting and combined treatment with an Mcl-1 inhibitor, improve CAR T-cell responses.
Significance: We demonstrate that DLBCL cells surviving CD19 CAR T-cell treatment develop a resistance phenotype with a “resistance signature” predictive of clinical CAR T-cell response, mediating cross-resistance between CAR T cells targeting different antigens. Our findings suggest that up-front dual-antigen targeting and combination therapies could improve clinical outcomes.
This article was highlighted in the November issue, and was one of two studies examining resistance to CAR-T or bispecific antibody therapy featured on the cover. The other article looked at the timing of genetic antigen loss over the course of CAR-T or bispecific antibody therapy.
Journal: Cancer Discovery
Sucralose Consumption Ablates Cancer Immunotherapy Response through Microbiome Disruption
Gut microbiota composition is directly associated with response to immunotherapies in cancer. The impact of diet on the gut microbiota and downstream immune responses to cancer remains unclear. In this study, we show that consumption of a common nonnutritive sweetener, sucralose, modifies microbiome composition, restricts T-cell metabolism and function, and limits immunotherapy response in preclinical models of cancer and patients with advanced cancer treated with anti–PD-1–based immune checkpoint inhibitors. Sucralose consumption is associated with a reduction in microbiota-accessible arginine, and amino acid supplementation or fecal microbiome transfer from anti–PD-1 responder mice completely restores T-cell function and immunotherapy response. Overall, sucralose consumption destabilizes the gut microbiota, resulting in compromised T-cell function and ablated immune checkpoint inhibitor response in cancer.
Significance: This study highlights an unappreciated role of sucralose in reducing immunotherapy efficacy in both mouse models and samples from patients with cancer through shifts in the microbiome and arginine degradation that lead to T-cell exhaustion. T-cell function and immunotherapy responses are restored through amino acid supplementation.
This article was highlighted in and featured on the cover of the November issue, which also included a related commentary. For more about the gut microbiome and how it could instead be modified to help enhance immunotherapy, check out this blog post.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Background: Social adversity from neighborhood disadvantage (ND) is associated with shorter breast cancer survival. Although studies have identified associations between ND and DNA methylation (DNAme) or gene expression (mRNA), a study integrating DNAme and mRNA to understand how the epigenome regulates key biological pathways in ND merits further inquiry.
Methods: DNAme-, mRNA-, miRNA-, and tRNA-derived fragment data were analyzed from 80 estrogen receptor+/HER2− breast cancer samples. We analyzed the association among ND, DNAme, coding, and noncoding data to understand how the epigenome regulates biological pathways.
Results: Twenty-five patients lived in ND and 55 in neighborhood advantage. In patients from ND, calcium signaling and cell adhesion pathways were hypermethylated, immune-related pathways hypomethylated, immune response genes upregulated, and estrogen response genes downregulated. Small RNA analysis showed differential expression of miRNA isoforms and tRNA-derived fragments related to ND. Subjective ND further correlated with epigenetic changes in calcium signaling, cell adhesion, and metabolic pathways, along with upregulation of proliferative and stemness pathways.
Conclusions: We discovered novel associations between ND and epigenomic regulation of clinically relevant oncogenesis pathways associated with aggressive biology, such as estrogen response pathways. These findings lay the foundation for multi-institutional studies to validate our findings.
Impact: This study demonstrates that ND, which is associated with shorter breast cancer survival, is also associated with epigenetic reprogramming of key oncogenesis pathways. These findings highlight pathways through which ND may affect tumor biology and lay the foundation for cancer control policy and behavioral interventions that may reverse the negative effects of ND on cancer biology.
The article was highlighted in the November issue.
Journal: Cancer Immunology Research
Chimeric antigen receptor (CAR) T-cell therapy has demonstrated remarkable efficacy against hematologic malignancies but has struggled to achieve comparable success in solid tumors. A key obstacle in solid tumors is the extracellular matrix (ECM), which impedes CAR T-cell infiltration. In clinical trials, neuroblastoma has shown responsiveness to GD2-directed CAR T-cell therapy; however, the failure of GD2.CAR T cells to effectively clear bulky disease—characterized by dense ECM—highlights the critical challenge of infiltration. In this study, we demonstrate that GD2.CAR T cells exhibit a unique infiltration restriction compared with other CAR T cells and endogenous T cells. A separate analysis of clinical datasets identified MMP7 and SPP1 [which encodes osteopontin (OPN)] as candidate genes to improve the infiltration of GD2.CAR T cells as these were upregulated in tumor-infiltrating leukocytes. MMP-7 and OPN overexpression enhanced CAR T-cell extravasation and interstitial movement in ECM-dense environments in vitro. Overexpression of either OPN or MMP-7 significantly improved tumor infiltration in a xenograft model of neuroblastoma. This resulted in improved tumor control and a survival extension in OPN-GD2.CAR T cell–treated mice compared with unmodified GD2.CAR T cells. OPN overexpression did not increase off-target infiltration into healthy tissues or promote tumor metastasis, highlighting its potential for safe therapeutic application. Our study provides a framework for further exploration of gene modifications to improve CAR T-cell infiltration in solid tumors and identifies OPN as a candidate to explore in this regard.
A related commentary was published in the November issue.
Journal: Cancer Prevention Research
Butyrate may reduce the risk of colorectal cancer and can be delivered to the colon using butyrylated high-amylose maize starch (HAMSB). This trial evaluated the effects of HAMSB on polyp burden in participants with familial adenomatous polyposis. This study was a randomized, double-blind, placebo-controlled crossover trial. In three 6-month periods, participants ingested 40 g/day of HAMSB or low-amylose starch, followed by the alternative, and then a washout. Participants underwent four video-recorded colonoscopies to assess polyp burden as the primary endpoint. At baseline, two distal bowel tattoos were placed: tattoo one where polyps were cleared at each scope and tattoo two where polyps were left in situ. Generalized linear mixed models were used to estimate the ratio of mean polyp counts in intervention compared with placebo periods. Seventy-two participants were randomized (33 female), with 49 completing the study. In the intention-to-treat analysis, HAMSB did not reduce mean global [0.9 fold change (FC); 95% confidence interval (CI), 0.77–1.06; P = 0.218] or small (<2.4 mm) polyp numbers (0.88 FC; 95% CI, 0.71–1.1; P = 0.267). There was a trend for the reduction of small polyps in tattoo one (0.72 FC; 95% CI, 0.5–1.03; P = 0.074). In the per-protocol analysis, there was a strong trend for HAMSB to reduce mean global small polyp numbers (0.79 FC; 95% CI, 0.62–1; P = 0.051). HAMSB may reduce polyp initiation in the distal bowel without causing regression or growth of existing polyps. However, 95% CIs indicate large uncertainty to the true direction of the treatment effect, and the P values provide only weak evidence against the null hypothesis of no treatment effect.
Prevention Relevance: There is convincing evidence that dietary fiber reduces the risk of colorectal cancer possibly by production of butyrate during microbial fermentation of indigestible fiber. This study was designed to determine if a dietary supplement that delivers butyrate to the colon reduces polyp burden in participants with familial adenomatous polyposis.
Journal: Cancer Research (November 1 issue)
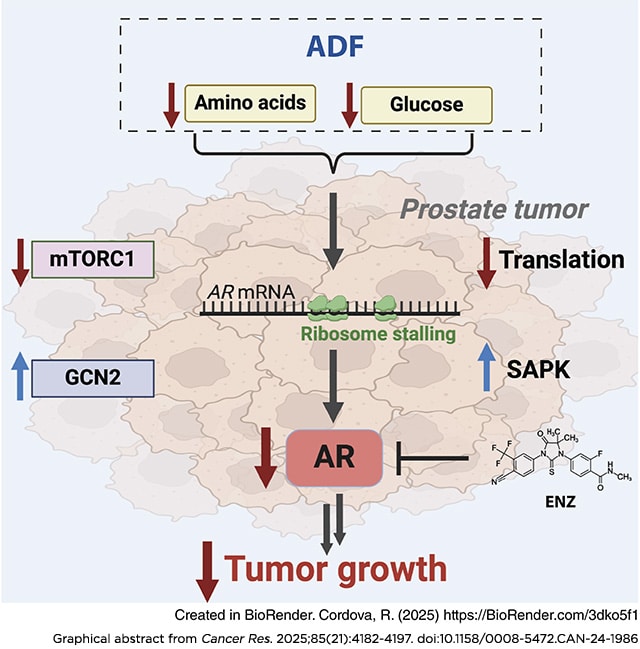
Epidemiologic studies suggest that diet can affect the incidence, progression, and response to treatment in multiple cancers, including prostate cancer. In this study, we investigated the use of dietary interventions, specifically caloric or protein restriction, in combination with antiandrogen therapy as a treatment for prostate cancer. Caloric restriction through alternate-day fasting (ADF) reduced androgen receptor (AR) expression and signaling. This reduction in AR enhanced the antitumor activity of the AR antagonist enzalutamide in multiple mouse models of prostate cancer. Mechanistic studies revealed that nutrient starvation via ADF predominantly decreased AR mRNA translation at the elongation stage due to AA limitation. Pharmacologic agents that similarly impair translation elongation and promote ribosome collisions mimicked the AR translation reduction observed with ADF. Overall, these findings suggest that AA limitation through ADF impairs translation elongation in prostate cancer, to which AR mRNA translation is susceptible, leading to a reduction in AR protein levels and enhancing AR-targeted therapy.
Significance: Fasting-induced caloric restriction reduces androgen receptor translation and enhances the activity of enzalutamide, suggesting that dietary intervention could be an effective strategy to enhance prostate cancer sensitivity to antiandrogen therapy.
A related commentary was published in the November 1 issue.
Journal: Cancer Research (November 15 issue)
γδ T cells can kill cancer cells via antibody-independent cytotoxicity (AIC) and antibody-dependent cellular cytotoxicity (ADCC). A better understanding of how these cytotoxic mechanisms are affected by different cancer cells and different T-cell donors could help identify improved immunotherapeutic strategies. To test the combinatorial interactions among T cell interdonor heterogeneity, cancer cell intertumor heterogeneity (ITH), and multimodal γδ T-cell killing, we performed a systematic single-cell phenoscaping analysis of more than 1,000 γδ T-cell and colorectal cancer patient-derived organoid cultures. Single-cell analysis of posttranslational modification (PTM) signaling, cell cycle, apoptosis, and T-cell immunophenotypes revealed that whereas unmodified γδ T cells have limited antitumor activity, IL15Rα–IL15 fusion protein [stabilized IL15 (stIL15)]-engineered γδ T cells can kill patient-derived organoids via AIC without exogenous cytokine support. However, when stIL15 γδ T cells only killed via AIC, cancer cells reciprocally rewired γδ T-cell PTM signal networks in an ITH-specific manner to suppress anticancer cytotoxicity. stIL15 γδ T cells could overcome this cancer cell immunomodulation by also engaging B7-H3–targeted ADCC independent of B7-H3 checkpoint activity. Combined AIC and ADCC rescued γδ T-cell PTM signaling flux and enabled γδ T cells to kill chemorefactory revival colon cancer stem cells. Together, these results demonstrate that multimodal γδ T-cell cytoxicity mechanisms can overcome ITH-specific immunomodulation to kill chemorefractory cancer cells.
Significance: Single-cell phenoscaping of more than 1,000 γδ T-cell and patient-derived organoid cultures shows that cancer cells suppress anticancer γδ T-cell cytotoxicity but γδ T cells can use multimodal killing to overcome immunomodulation.
Journal: Clinical Cancer Research (November 1 issue)
Purpose: Izalontamab (SI-B001) is a novel EGFR×HER3 bispecific antibody. This first-in-human phase I study presents the safety and pharmacokinetics of izalontamab.
Patients and Methods: Previously treated patients with locally advanced or metastatic epithelial tumors were enrolled in the dose-escalation or dose-expansion phases. The dose-escalation phase consisted of an accelerated titration and a “3 + 3” design with nine dose levels from 0.4 to 28.0 mg/kg. The dose-expansion phase included five dose levels from 6.0 to 21.0 mg/kg. Izalontamab was administered intravenously weekly or every 2 weeks in a 4-week cycle. Available pretreatment specimens were obtained to explore the relationship between EGFR/HER3 expression and efficacy.
Results: Sixty patients were enrolled. Among the 60 enrolled patients, 49 had non–small cell lung cancer (NSCLC), 6 had nasopharyngeal cancer, 3 had head and neck cancer squamous cell carcinoma, and 2 had other types of cancer. The most common treatment-related adverse events were rash (42%), paronychia (25%), and infusion-related reactions (23%). No drug-related death occurred. Izalontamab displayed a nonlinear pharmacokinetic behavior, and clearance at steady state seemed to be approaching a dose-independent value at 6 mg/kg and above. The best response included two confirmed partial responses in patients with NSCLC and head and neck cancer squamous cell carcinoma; 18 patients had stable disease, including NSCLC (n = 17) and colorectal cancer (n = 1). The recommended phase II dose for izalontamab was determined as 9 to 16 mg/kg weekly.
Conclusions: Izalontamab was well tolerated and demonstrated preliminary antitumor activity in patients with locally advanced or metastatic epithelial tumors, supporting it as a promising therapeutic candidate for combination therapies, with a phase III study currently underway.
Journal: Clinical Cancer Research (November 15 issue)
Purpose: To evaluate the safety, pharmacokinetics, pharmacodynamics, and efficacy of ficerafusp alfa (BCA101), a first-in-class bifunctional protein targeting EGFR and TGF-β, as monotherapy and in combination with pembrolizumab in patients with advanced solid tumors.
Patients and Methods: At escalating doses in a parallel 3 + 3 design, patients with EGFR-driven advanced solid tumors received weekly intravenous ficerafusp alfa as monotherapy (64–1,500 mg) or in combination (240–1,500 mg) with pembrolizumab (200 mg i.v. every 3 weeks). The primary objective was to determine safety/tolerability. Secondary objectives included assessment of pharmacokinetics, immunogenicity, and preliminary efficacy per investigator-assessed response. Exploratory analyses included pharmacodynamic biomarkers.
Results: Among 61 patients (monotherapy, n = 46; combination, n = 15), the most common treatment-related adverse events included acneiform dermatitis (46%) and fatigue (20%) with monotherapy and acneiform dermatitis (73%), fatigue (53%), pruritus (40%), epistaxis (40%), and maculopapular rash (40%) with combination therapy. One patient had a dose-limiting toxicity with 1,250-mg monotherapy (grade 3 anemia and hematuria). The MTD was not reached in either cohort. With monotherapy, objective response was observed in one of 42 evaluable patients and 16 (38%) achieved stable disease. With combination therapy, four of 13 evaluable patients (31%) had a confirmed response, including one with head and neck squamous cell carcinoma refractory to anti–PD-1 therapy and cetuximab. Prolonged neutralization of plasma TGF-β1 was observed at doses ≥500 mg.
Conclusions: Ficerafusp alfa exhibited a manageable safety profile and clinical activity as monotherapy and in combination with pembrolizumab, with exposure increasing proportionally at anticipated therapeutic doses.
A related commentary was published in the November 15 issue.
Journal: Molecular Cancer Research
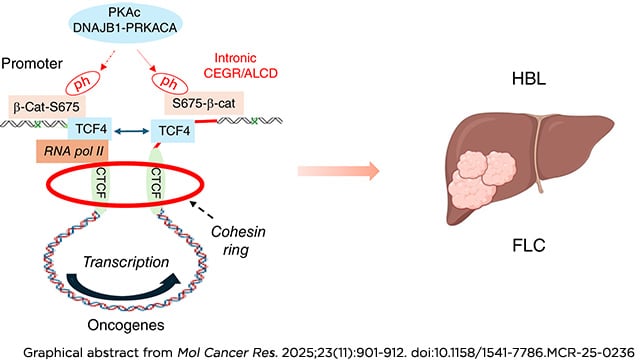
The pediatric and adolescent liver cancers, hepatoblastoma (HBL) and fibrolamellar hepatocellular carcinoma (FLC), respectively, are dangerous diseases requiring aggressive surgery, when feasible, and nontargeted toxic chemotherapy for a chance of cure due to insufficient knowledge of underlying molecular mechanisms. We have previously reported the essential role of ph-S675-β-catenin in the reorganization of genomic structure in HBL and FLC by oncogenic activation via chromosomal regions called cancer-enhancing genomic regions or aggressive liver cancer domains (CEGR/ALCD). In FLC, the fusion DNAJB1-PKAc (J-PKAc) oncoprotein phosphorylates β-catenin at Ser675, triggering such CEGRs/ALCDs-mediated activation of oncogenes. In this study, we found that all members of the cohesin ring—CTCF, Rad21, SMC1, SMC3, and STAG1—and β-catenin–TCF4 are bound to CEGRs/ALCDs of oncogenes in HBL and FLC, as well as many other cancers, and that this binding increases transcription. Examination of a large cohort of HBL and FLC samples revealed that cohesin ring expression is dramatically elevated in the majority. The cohesin ring, as well as the ph-S675-β-catenin–TCF4–p300 complex, is detected on both the promoter and intron-located CEGRs/ALCDs of NRF2 and Thy1, correlating with increased transcription. This suggests that the cohesin ring creates the DNA loop for oncogene activation. The inhibition of the cohesin ring by JQ1 reduces the proliferation of HBL and FLC cells in culture, as well as cells expressing the FLC-specific J-PKAc fusion oncogene.
Implications: These studies provide evidence that J-PKAc–β-catenin and the cohesin ring cooperate in oncogenic activation for both HBL and FLC.
This article was highlighted in the November issue.
Journal: Molecular Cancer Therapeutics
TUB-040 is a highly homogeneous and hydrophilic antibody–drug conjugate (ADC) targeting sodium-dependent phosphate transport protein 2b (NaPi2b), a surface receptor overexpressed in ovarian cancer and non–small cell lung adenocarcinoma. Previous NaPi2b-directed therapies have shown target-mediated and expression-dependent clinical activity. However, none of the previous tubulin inhibitor-based ADCs have been able to leverage the full therapeutic potential of the target. TUB-040 was constructed with a drug-to-antibody ratio of 8 using the Tubutecan linker-payload technology based on ethynylphosphonamidates (P5 conjugation chemistry), a protease cleavage site, and exatecan, a potent topoisomerase 1 inhibitor. TUB-040 induces potent antigen-specific cytotoxicity against NaPi2b-expressing cancer cells and demonstrates strong bystander activity. It displays favorable pharmacokinetic behavior, showing dose proportionality and superimposable total antibody and intact ADC curves, as well as low free payload levels, reflecting the high stability of TUB-040 enabled by the P5 conjugation platform. This specific feature also ensures sustained delivery of exatecan to tumor sites, which translates into excellent in vivo efficacy and tolerability. In cell line– and patient-derived xenograft models, including those with low target expression, single-dose TUB-040 administration leads to prolonged tumor growth inhibition and significant rates of complete remission, with a minimally effective dose level of 1 mg/kg in the OVCAR-3 model. Repeated-dose toxicologic assessment in rats indicates that TUB-040 is well tolerated, with no evidence of lung toxicity or thrombocytopenia. Taken together, TUB-040 is designed to enable long-lasting, durable tumor responses and to optimize both efficacy and tolerability, supporting the advancement of TUB-040 into clinical trials.
This article was highlighted in the November issue.
Journal: Cancer Research Communications
Auranofin Synergizes with Cisplatin in Reducing Tumor Burden of NOTCH-Dependent Ovarian Cancer
The NOTCH pathway regulates cell proliferation, differentiation, and stem cell maintenance. Thus, aberrant NOTCH activation plays a key role in cancer initiation, progression, and chemoresistance. Mutations and amplification of NOTCH pathway genes have been identified in high-grade serous ovarian cancers and are associated with poor clinical outcomes. Among the four NOTCH receptors, NOTCH3 alterations were strongly correlated with poor overall survival. Previously, we identified auranofin, an oral gold salt therapeutic compound, as a novel NOTCH pathway inhibitor that disrupts the DNA binding of RBPJ, the major downstream transcriptional effector of the NOTCH pathway. In this study, we surveyed the response of eight ovarian cancer cell lines to auranofin and found IC50 values ranging from 1.7 to 12 μmol/L, with NOTCH3-negative SKOV3 cells having the highest IC50 value. In NOTCH-dependent OVCAR3 cells, auranofin synergized with cisplatin to enhance cell death. Importantly, auranofin treatment led to a dose-dependent decrease in RBPJ occupancy at the NOTCH-dependent promoters, HES1 and HES4. Furthermore, knocking down NOTCH3 in OVCAR3 cells significantly decreased sensitivity to auranofin, further supporting the notion that NOTCH3 signaling is a major target of auranofin. Moreover, auranofin increased cisplatin efficacy in an OVCAR3-derived xenograft mouse model. Using eight patient-derived cancer organoid models, we found that auranofin increased cisplatin efficacy in killing cancer organoids generated from clinically platinum-sensitive patients but also restored platinum response in a subset of organoid models developed from platinum-resistant patients. These studies underscore the potential of auranofin to improve platinum-based cancer therapy, particularly in NOTCH3-expressing cancers.
Significance: NOTCH signaling underlies cancer initiation, progression, and chemoresistance. Our study revealed the potential of auranofin as a NOTCH pathway inhibitor to enhance the efficacy of platinum-based ovarian cancer therapy.