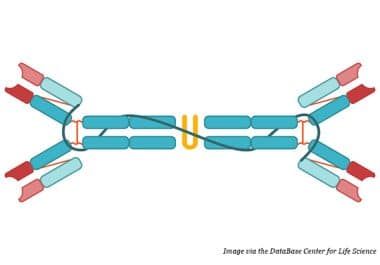
Dragging Oncogenes From the Cell: A New Horizon for Targeting KRAS
This Trojan horse-like treatment strategy may help neutralize undruggable cancer drivers.


This Trojan horse-like treatment strategy may help neutralize undruggable cancer drivers.
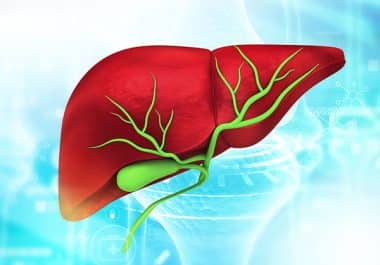
Researchers are finding early clinical success in developing more targeted treatments for bile duct cancer, also called cholangiocarcinoma.

During late spring and early summer, the U.S. Food and Drug Administration (FDA) approved four new molecularly targeted therapeutics—alpelisib...

On Monday, the U.S. Food and Drug Administration (FDA) announced the highly anticipated approval of the molecularly targeted therapeutic...

Last week saw a flurry of new anticancer therapeutics approved by the U.S. Food and Drug Administration (FDA) for...

Recently, the U.S. Food and Drug Administration (FDA) approved the molecularly targeted therapeutic vemurafenib (Zelboraf) for treating certain adults...

Last week, the U.S. Food and Drug Administration (FDA) approved the molecularly targeted therapeutic acalabrutinib (Calquence) for treating adults...

This week’s excitement surrounding the groundbreaking U.S. Food and Drug Administration (FDA) approval of the CAR T–cell therapy tisagenlecleucel...

Earlier this week, the U.S. Food and Drug Administration (FDA) approved a new molecularly targeted therapeutic called neratinib (Nerlynx)...
recent data suggest that there is another potential contender for a biomarker-based FDA approval: a targeted therapeutic called larotrectinib...