H. Pylori Infection Alters the Trajectory of Gastric Cancer Beyond Initiation
By Laura Romano, PhD
Gastric cancer is among the top five most common cancers globally, with approximately 1 million new cases diagnosed annually (1). One well known risk factor for gastric cancer is Helicobacter pylori (Hp) infection, a bacterium that drives gastric inflammation and is found in 50% of the world’s population (2). However, the exact mechanisms of Hp-driven gastric cancer are not well defined. A recent study published in Cancer Research Communications sheds light on this by identifying the expansion of a population of precancerous gastric epithelial cells, driven by Hp-mediated inflammation, in a genetically triggered background of metaplasia (pre-cancer) (3).
The study was led by Valerie O’Brien, PhD, then a Postdoctoral Fellow in the laboratory of Nina Salama, PhD, at Fred Hutchinson Cancer Center in Seattle, Washington. As a postdoc, Dr. O’Brien was awarded the 2018 Debbie’s Dream Foundation-AACR Gastric Cancer Research Fellowship, in memory of Sally Mandel, for her project titled “Assessing Helicobacter pylori contributions to stomach cancer.” It is commonly believed that Hp infection primarily plays a role in initiating gastric cancer, as many individuals with this disease clear their Hp infection at some point. However, some individuals with gastric cancer retain their Hp infection, which could give the bacterium an opportunity to impact the later stages of disease. The findings in Dr. O’Brien’s Cancer Research Communications publication provide evidence that Hp infection is not only involved in initiating gastric cancer but may also alter later disease trajectory by inducing expansion of precancerous gastric epithelial cells.

Critical to the study was the use of a mouse model with a constitutively active Kras allele (KrasG12D) in gastric chief cells, the cells responsible for producing the stomach’s digestive enzymes. Inducing constitutively active KRAS in chief cells rapidly drives metaplasia in the stomach (4). Dr. O’Brien and colleagues previously found that Hp infection could synergize with constitutively active KRAS, resulting in severe inflammation, striking tissue changes, and greater dysplasia (5). Using a combination approach of Hp infection and KRAS activation (Hp+, KRAS+) in mice paved the way for the investigators to delineate the Hp-driven disease trajectory.
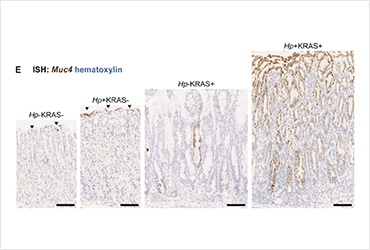
Using stomach tissue from these mice, Dr. O’Brien and colleagues performed single-cell RNA sequencing (scRNA-seq) to uncover the transcriptomic profiles of gastric epithelial cells. scRNA-seq followed by cluster analyses revealed a striking expansion of cells genetically similar to pit cells, mucus-producing cells normally found at the top of the gastric epithelium, in Hp+KRAS+ mice. Gene ontology analysis suggested these pit cell clusters were involved in pathways related to cancer development, such as cellular remodeling and epithelial-mesenchymal transition. Moreover, these pit cell clusters expressed metaplasia-related genes, leading the authors to coin the term “metaplastic pit cells” to describe the gastric epithelial cells that expanded upon Hp infection. Furthermore, the investigators identified the intestinal mucin gene, Muc4, as a proxy marker for metaplastic pit cells, thereby allowing them to tease apart the factors driving expansion of these cells in Hp+KRAS+. Using in situ hybridization, it was shown that Hp-driven inflammation was required for expansion of Muc4-expressing metaplastic pit cells.
While the mouse model provided strong evidence of Hp-driven expansion of Muc4-expressing metaplastic pit cells, Dr. O’Brien’s work sought to validate these results using human samples. Immunostaining analysis revealed that clinical gastric cancer samples expressed metaplastic pit cells, as determined by the presence of MUC4. Moreover, the researchers also stained these clinical samples for Ki-67, a cell proliferation marker, and found that individual cells expressed both MUC4 and Ki-67, suggesting that metaplastic pit cells are proliferative. Analyzing available scRNA-seq data for human samples from other studies, the researchers found that MUC4 levels were significantly higher in stage 4 tumors compared to stage 1 tumors, suggesting that further expansion of metaplastic pit cells occurs as gastric cancer disease progresses.
Dr. O’Brien’s work demonstrates that in mice with genetically triggered metaplasia in the stomach, Hp infection may alter disease trajectory through induced expansion of unique proliferative cell populations. However, as Dr. O’Brien notes, “we still have much to learn about how and why Hp infection leads to gastric cancer. In our mouse model, metaplastic pit cells required Hp-driven inflammation, and I am particularly excited to investigate how the immune response to Hp may harm the stomach epithelium.”
After being awarded the 2018 Debbie’s Dream Foundation-AACR Gastric Cancer Research Fellowship, in memory of Sally Mandel, Dr. O’Brien secured additional funding from other organizations to jumpstart her career as an independent investigator. “I am so grateful to the AACR for their support. I am proud to be an AACR member and will continue publishing my best work in AACR journals,” Dr. O’Brien commented. She now runs an independent laboratory at Purdue University’s College of Pharmacy, where her research group will continue to explore mechanisms of Hp-driven gastric cancer and will work on developing therapeutics to combat this disease.
References
- Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: a population-based modelling study. EClinicalMedicine 2022;47;101404. doi: 10.1016/j.eclinm.2022.101404.
- Salvatori S, Marafini I, Laudisi F, Monteleone G, Stolfi C. Helicobacter pylori and gastric cancer: pathogenetic mechanisms. Int. J. Mol. Sci. 2023;24;2895. doi: 10.3390/ijms24032895.
- O’Brien VP, Kang Y, Shenoy MK, Finak G, Young WC, Dubrulle J, et al. Single-cell profiling uncovers a Muc4-expressing metaplastic gastric cell type sustained by Helicobacter pylori-driven inflammation. Cancer Res. Commun. 2023;3;1756-1769. doi: 10.1158/2767-9764.CRC-23-0142.
- Choi E, Hendley AM, Bailey JM, Leach SD, Goldenring JR. Expression of activated Ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology. 2016;150;918-930. doi: 10.1053/j.gastro.2015.11.049.
- O’Brien VP, Koehne AL, Dubrulle J, Rodriguez AE, Leverich CK, Kong VP, et al. Sustained Helicobacter pylori infection accelerates gastric dysplasia in a mouse model. Life Sci. Alliance. 2020;4; e202000967. doi: 10.26508/lsa.202000967.