Editors’ Picks: Highlights from the AACR Journals
As you snack on turkey sandwiches and leftover pumpkin pie, check out the articles highlighted by the editors of the 10 AACR journals for the month of November. This month, the selected articles include results from five clinical trials, a technological tool for predicting cancer survival trends, and an imaging-based platform for studying the tumor immune microenvironment.
As always, the articles are freely available online for a limited time.
Journal: Clinical Cancer Research (November 1 issue)
Patients with estrogen receptor (ER)-positive breast cancer are typically treated with endocrine therapy, but treatment resistance is common. To help prevent or overcome resistance, endocrine therapies are often combined with inhibitors of CDK4/6 or PI3K. Fulvestrant (Faslodex), the only approved selective estrogen receptor degrader (SERD) to date, has shown clinical benefit in patients with ER-positive breast cancer, but its poor oral bioavailability may limit its clinical activity. Here, the authors conducted a phase I clinical trial to evaluate the safety and efficacy of LSZ102, an investigational oral SERD, alone or in combination with the CDK4/6 inhibitor ribociclib (Kisqali) or the PI3K inhibitor alpelisib (Piqray) in patients with ER-positive breast cancer. Objective responses were observed in 1.3 percent of 77 patients treated with LSZ102 alone, 16.9 percent of 78 patients treated with LSZ102 and ribociclib, and 7 percent of 43 patients treated with LSZ102 and alpelisib. Serious treatment-related adverse events occurred in 10 percent of patients and were most common among patients treated with LSZ102 and alpelisib. The authors conclude that LSZ102 was well tolerated alone and in combination with ribociclib and showed preliminary clinical activity in combination with ribociclib or alpelisib. This article is highlighted in the November issue.
Journal: Cancer Discovery
Malignant diseases within the pleural cavity, such as malignant pleural mesothelioma (MPM) and metastases from lung and breast cancers, are aggressive and respond poorly to therapies. Chimeric antigen receptor (CAR) T cells targeting mesothelin, an antigen overexpressed in a broad range of solid tumors, and delivered to the pleural cavity have shown promise in preclinical models. In this first-in-human phase I trial, the authors showed that this approach was safe and well tolerated. Based on the observation that anti–PD-1 therapy could rescue the function of exhausted CAR T cells in the mouse model, the investigators also evaluated the combination of the immune checkpoint inhibitor pembrolizumab (Keytruda) with intrapleural administration of mesothelin-targeting CAR T cells in a subcohort of patients. Among those patients, the median overall survival from CAR T-cell infusion was 23.9 months, one-year overall survival was 83 percent, and persistence of functional CAR T cells was observed. This article was featured on the cover, in a commentary, and is highlighted in the November issue.
Journal: Cancer Epidemiology, Biomarkers and Prevention
Characterizing Trends in Cancer Patients’ Survival Using the JPSurv Software
Researchers assess the trends in cancer survival by comparing survival rates between grouped years of diagnosis. The authors of this article developed a webtool called JPSurv that analyzes survival data by single year of diagnosis and estimates changes in survival trends and year-over-year trend measures. JPSurv was applied to analyze relative survival data from the Surveillance, Epidemiology, and End Results (SEER) Program for individuals diagnosed with female breast cancer, melanoma, non-Hodgkin lymphoma (NHL), and chronic myeloid leukemia (CML) between 1975 and 2015. This analysis captured the substantial improvement in relative survival since the mid-1990s for all cancer sites. The most significant improvements in five-year relative survival were observed for metastatic melanoma after 2009, CML in 1995–2010, and NHL in 1995–2003, and coincided with the introduction of new treatments for these diseases. This study showed the value of JPSurv for estimating and interpreting cancer survival trends, complementing other traditional survival analyses. This article is highlighted in the November issue.
Journal: Cancer Prevention Research
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related death. Unfortunately, most PDAC cases are diagnosed only after the disease has advanced, when treatment is more difficult and less likely to succeed. Carriers of a pathogenic or likely pathogenic variant in the BRCA1, BRCA2, ATM, and/or PALB2 genes are at increased risk of developing PDAC, but current guidelines recommend surveillance only for carriers who also have a family history of the disease. In this study, the authors conducted a retrospective analysis to evaluate the utility of endoscopic ultrasound-based surveillance (EUS) for PDAC in 64 carriers of pathogenic/likely pathogenic variants who did not have a family history of PDAC. Most of the carriers had a BRCA2 variant and had a personal history of cancer other than PDAC. Among the 64 carriers, pancreatic abnormalities, including cysts, masses, and luminal abnormalities, were detected by EUS in 44 percent of individuals, with two individuals developing PDAC during surveillance. No serious complications arose from surveillance. The authors conclude that PDAC surveillance should be considered but that further study is needed to understand its effectiveness among carriers of pathogenic/likely pathogenic variants who do not have a family history of PDAC.
Journal: Clinical Cancer Research (November 15 issue)
Chronic myelomonocytic leukemia (CMML) is a rare cancer, officially characterized as an overlap myelodysplastic/myeloproliferative neoplasm syndrome. CMML tumors are frequently driven by the cytokine GM-CSF, which is activated by the kinase JAK2. In this phase I/II clinical trial, the researchers investigated the safety and efficacy of the JAK inhibitor ruxolitinib (Jakafi) in treating patients with CMML. Because researchers often face difficulties achieving sufficient statistical power to secure FDA approval in clinical trials of rare diseases due to the limited number of patients, during this trial, the researchers used tumor biopsy tissue taken from 13 study participants to develop patient-derived xenograft models in mice engineered to secrete human cytokines. Xenografted mice were treated with either ruxolitinib or a vehicle control, and the responses of these xenografts to ruxolitinib were monitored in tandem with patient responses. In human patients, ruxolitinib was well tolerated, with each grade 3/4 adverse event occurring in 10 percent or fewer of patients. Thirty-eight percent of patients experienced a partial or complete response, and an additional 44 percent experienced stable disease. This translated to a median overall survival of 28.8 months in patients whose tumors responded, compared with 22.3 months in patients with stable or progressive disease. Among mice xenografted with responsive tumors, those treated with ruxolitinib experienced an overall survival 11 days longer than their vehicle-treated counterparts, while the same was not true of mice xenografted with tumors from patients who did not respond. The authors suggested that patient-derived models may provide robust and accurate data to support clinical studies with small numbers of participants. This study was highlighted in the November 15 issue, and a related commentary is available here.
Journal: Cancer Research (November 1 issue)
Macrophage-Derived Cholesterol Contributes to Therapeutic Resistance in Prostate Cancer
Prostate cancer is often driven by androgen receptor (AR) activity and is commonly treated with drugs that block AR signaling. However, most prostate tumors eventually become resistant to androgen blockade—a process known as castration resistance—and patients are required to switch to other treatments. While mechanisms of AR resistance have been extensively studied in the context of tumor cells, the authors of this study wanted to understand the role of the tumor microenvironment—specifically tumor-associated macrophages (TAMs), which have been implicated in resistance and poor prognosis. Here, the researchers developed mouse prostate cancer cell lines resistant to the antiandrogen leuprorelin (Lupron) and observed high levels of macrophage infiltration in tumors resulting from injection of these cells into mice. They found that depleting macrophages from these tumors decreased the intra-tumor synthesis of steroid hormones such as androgen and sensitized the tumors to antiandrogen therapy. Further, macrophage depletion decreased AR activation (but not expression), and co-culture of prostate cancer cells with macrophages stimulated AR activation and protected cells from the effects of antiandrogen treatment. The researchers found that the macrophages were boosting AR activity by transferring cholesterol—a key precursor of androgen—to the tumor cells so that they could produce androgen and bypass chemical castration. Activation of the LXRβ receptor reversed macrophage-driven androgen activation, leading the researchers to speculate that LXRβ may be a promising therapeutic target in patients with castration-resistant disease. A commentary on this study is available here.
Journal: Cancer Research (November 15 issue)
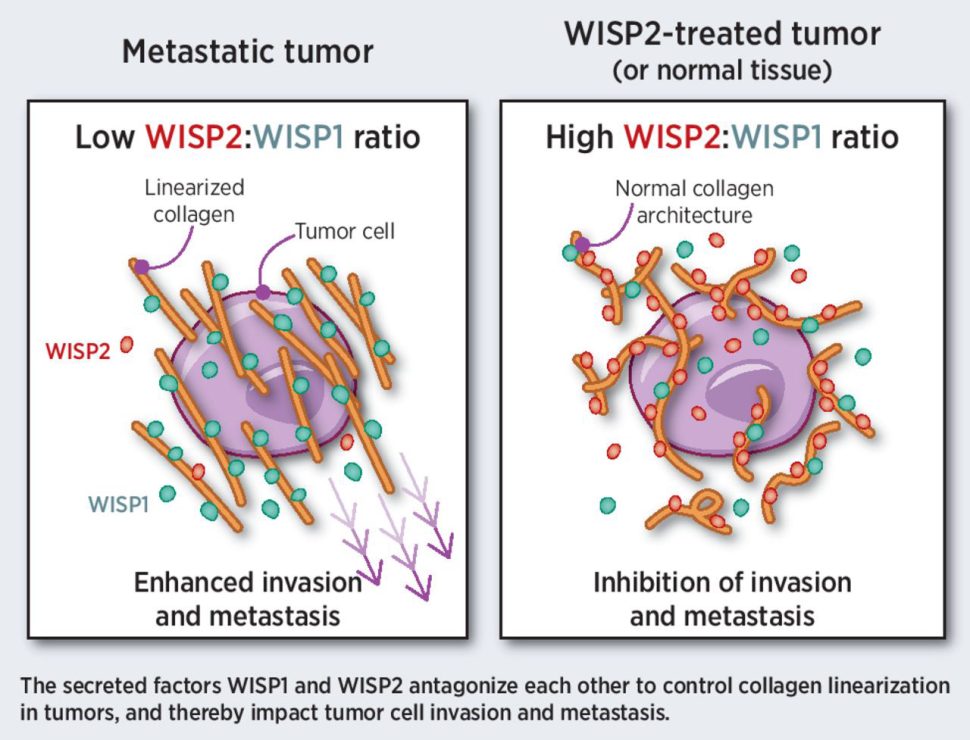
The linearization of collagen in primary tumors promotes invasion and metastasis and is associated with poor prognosis. The authors of this study previously demonstrated that the WISP1 protein binds type I collagen and promotes its linearization through a C-terminal domain. Prior research indicated that the related WISP2 protein lacks this C-terminal domain and is associated with good prognosis in patients. The authors hypothesized that WISP2 may antagonize the function of WISP1 and suppress collagen linearization. Consistent with this hypothesis, they observed that WISP2 prevented binding of WISP1 with type I collagen and inhibited linearization in in vitro assays. The authors also analyzed patient data, which revealed that WISP2 expression was lower in most solid tumors compared with normal tissue, suggesting a potential role for WISP2 loss in cancer development or progression. Increasing WISP2 levels in breast cancer models impaired collagen linearization and prevented tumor cell invasion and metastasis, highlighting WISP2 restoration as a potential therapeutic opportunity. The authors propose WISP2 as an endogenous inhibitor of collagen linearization that could be therapeutically restored to treat cancer. A related commentary can be found here.
Journal: Blood Cancer Discovery
Despite the therapeutic advancements in the treatment of acute myeloid leukemia (AML), some patients do not respond to therapy and others who initially respond eventually relapse. For patients with refractory or relapsed AML (R/R AML), limited therapeutic options are available and outcomes are dismal. Cytarabine is a primary component of the salvage chemotherapy regimens for R/R AML patients. AML patients carry immune aberrations that lead to immune suppression, and studies in preclinical models have shown that PD-1–expressing CD8+ T cells and regulatory T cells accumulate during disease progression, leading to T-cell exhaustion. Furthermore, restoration of T cell function was observed in patients who achieved complete remission after therapy, suggesting that T-cell exhaustion may be targeted with immune checkpoint blockade. This phase II study investigated whether pembrolizumab (Keytruda) following high-dose cytarabine (HiDAC) chemotherapy improves the outcomes in R/R AML patients. The authors reported that the addition of pembrolizumab to HiDAC led to overall response rate, composite complete remission rate, and median overall survival of 46 percent, 38 percent, and 11.1 months, respectively. The treatment was tolerable, with self-limiting adverse events. Furthermore, immunological and genomic profiling before and after therapy revealed potential biomarkers of response to pembrolizumab. This article is highlighted in the November issue and featured in a commentary.
Journal: Molecular Cancer Research
Hedgehog signaling has been linked to the initiation of medulloblastoma, the most common malignant brain tumor in children. The stem cell factor SOX9 is a downstream target of Hedgehog signaling and has been suggested to be a possible therapeutic target for the treatment of the Sonic Hedgehog-activated subtype of medulloblastoma (SHH-MB). However, the contribution of SOX9 in SHH-MB remains unclear. Using mouse models of SHH-MB, the authors of this study found that murine SHH-MBs had higher expression of SOX9 compared with several other medulloblastoma subtypes and that expression of SOX9 was widespread in SHH-MB tumors. In addition, analysis of human medulloblastoma data revealed the presence of three distinct SOX9 populations in SHH-MB but not in other subtypes. Despite the widespread expression of SOX9 in SHH-MB tumors, lower SOX9 expression was observed in Math1-positive granule cell precursors (GCPs), which are the initiating cells of SHH-MB. Moreover, ablation of SOX9 in GCPs did not impact the development of SHH-MB, suggesting that SOX9 is not required for Math1-positive GCP-driven tumor formation. Together, the results indicate that SOX9 may not be a viable therapeutic target for SHH-MB. This article is highlighted and featured on the cover of the November issue.
Journal: Cancer Immunology Research
Spatial UMAP and Image Cytometry for Topographic Immuno-oncology Biomarker Discovery
With the advent of immuno-oncology and the development of new therapies focused on potentiating the body’s anticancer immune responses, characterizing complex cell phenotypes and the spatial relationships that exist among tumor cells and immune cells within the tumor microenvironment has become increasingly critical. Multiplex immunofluorescence (mIF) technology enables the visualization of such spatial relationships while preserving the architectural features of the tumor. However, the analysis of mIF data can be complex and time-consuming. In this study, the authors used tumor tissue samples from 93 patients with metastatic melanoma to develop and validate an mIF data analysis pipeline based on image cytometry. They built a spatial uniform manifold approximation and projection (UMAP) algorithm to cluster the data and correlate them with prognostic factors. This approach revealed the presence of “immune neighborhoods” and associated patterns of protein expression. For example, the authors observed that the intensity of PD-L1 and PD-1 expression was spatially encoded, with the highest PD-L1 expression on CD163+ cells in neighborhoods with high CD8+ T cell density, and the highest PD-1 expression on CD8+ cells in neighborhoods with high density of tumor cells. These differences in spatial clustering correlated with the clinical outcomes, indicating the potential of this approach as a tool to visualize and interpret mIF data and identify prognostic and predictive immuno-oncology biomarkers. This article was featured on the journal’s cover.
Journal: Molecular Cancer Therapeutics
Tenosynovial giant cell tumors (TGCT)—malignant groupings of macrophages and other inflammatory cells—are largely driven by the activity of colony stimulating factor 1 receptor (CSF1R), which can suppress immune infiltration, promote angiogenesis, and activate bone-degrading osteoclasts. The CSF1R inhibitor pexidartinib (Turalio) is FDA approved for the treatment of TGCT, but pexidartinib causes many off-target toxicities due to targeting of several other kinases and production of hepatotoxic metabolites. In this study, researchers tested vimseltinib, a highly specific, orally bioavailable inhibitor of CSF1R. Vimseltinib inhibited the growth of mouse myelogenous leukemia cells with almost four-fold higher potency than pexidartinib at 1 percent of the dose, and also prevented differentiation of cultured osteoclast cells. Though it is difficult to model TGCT in mice, the researchers found that vimseltinib decreased the growth of colorectal cancer cell mouse xenografts by an average of 52 percent, which increased to 74 percent when used in combination with anti-PD-1 therapy. Vimseltinib also decreased tumor-associated macrophages by six-fold and blocked osteoclast-mediated bone degradation after the injection of prostate cancer cells into mouse tibias. The researchers also included preliminary results from a phase I/II clinical trial of vimseltinib in patients with TGCT. All three patients from the first cohort experienced partial responses and no grade 3+ toxicities after two 28-day treatment cycles, with improved responses at extended follow-up for two of the patients. The researchers speculate that vimseltinib may prove to be more effective and better tolerated than pexidartinib for the treatment of TGCT. This study was highlighted and featured on the cover of the November issue.