Editors’ Picks, April 2024: Targeting Metabolism, KRAS, and More
As April showers bring May flowers, the editors of the 10 American Association for Cancer Research (AACR) journals shower us with their picks of this month’s most blossoming studies.
For April, the editors selected two studies about metabolic interventions for acute myeloid leukemia, an analysis of how serum microRNAs (miRNAs) used for ovarian cancer screening might differ by race, a new inhibitor that acts upstream of KRAS, and more. The abstract of each highlighted study is included below, and each article is freely available for a limited time.
Journal: Blood Cancer Discovery
Rare preleukemic hematopoietic stem cells (pHSC) harboring only the initiating mutations can be detected at the time of acute myeloid leukemia (AML) diagnosis. pHSCs are the origin of leukemia and a potential reservoir for relapse. Using primary human samples and gene editing to model isocitrate dehydrogenase 1 (IDH1) mutant pHSCs, we show epigenetic, transcriptional, and metabolic differences between pHSCs and healthy hematopoietic stem cells (HSC). We confirm that IDH1-driven clonal hematopoiesis is associated with cytopenia, suggesting an inherent defect to fully reconstitute hematopoiesis. Despite giving rise to multilineage engraftment, IDH1-mutant pHSCs exhibited reduced proliferation, blocked differentiation, downregulation of MHC class II genes, and reprogramming of oxidative phosphorylation metabolism. Critically, inhibition of oxidative phosphorylation resulted in the complete eradication of IDH1-mutant pHSCs but not IDH2-mutant pHSCs or wild-type HSCs. Our results indicate that IDH1-mutant preleukemic clones can be targeted with complex I inhibitors, offering a potential strategy to prevent the development and relapse of leukemia.
Significance: A high burden of pHSCs is associated with worse overall survival in AML. Using single-cell sequencing, metabolic assessment, and gene-edited human models, we find human pHSCs with IDH1 mutations to be metabolically vulnerable and sensitive to eradication by complex I inhibition.
This study was featured on the cover of the March issue. A related commentary is available.
Journal: Cancer Discovery
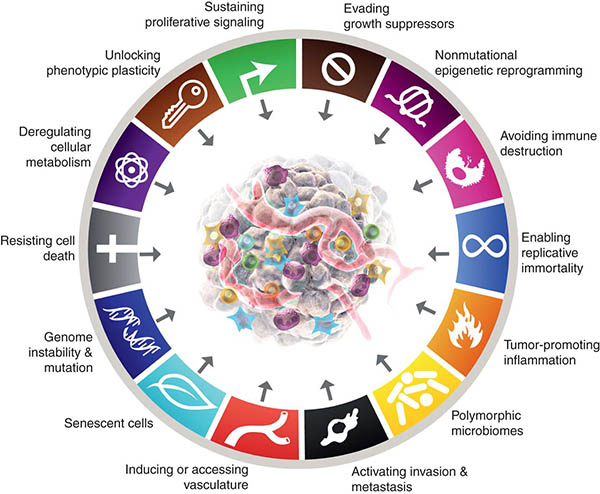
Cancer Hallmarks: Piecing the Puzzle Together
The initial Hallmarks of Cancer were first described in 2000 by Douglas Hanahan and Robert Weinberg as a set of six essential functional properties of tumors that are acquired in the process of malignant initiation and progression. Since then, this list has been expanded in 2011 and 2022 to include additional core hallmarks and defining characteristics that enable cancer cells to adopt these functional capabilities, deepening our understanding of the complexity of tumor biology. As we move forward to further comprehend the role of the hallmarks in cancer biology, we asked early- and mid-career investigators for their opinions on the key challenges and open questions related to each of the fourteen Cancer Hallmarks.
This article was part of a special community-driven issue of Cancer Discovery. For more about the issue, check out this blog post.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Background: Despite the various anticancer activities of tocopherols, little is known about tocopherols associated with lung cancer risk among low-income African Americans (AA) and European Americans (EA) who are disproportionately affected by the disease.
Methods: We conducted a nested case–control study that included 209 incident lung cancer cases and 406 matched controls within the Southern Community Cohort Study. Using biospecimens collected at cohort enrollment, plasma levels of α-, β/γ-, δ-, and total-tocopherols were measured by high-performance liquid chromatography with photodiode array detection. Conditional logistic regression was used to estimate ORs and 95% confidence intervals (CI) for lung cancer risk after adjusting for potential confounders. Stratified analyses were also conducted.
Results: Plasma levels of total-tocopherols were inversely associated with lung cancer risk overall [OR (95% CI) for the highest vs. lowest tertile = 0.51 (0.30–0.90)]. The inverse association remained significant among EAs [0.20 (0.06–0.65)], men [0.43 (0.21–0.90)], current smokers [0.49 (0.26–0.93)], and cases diagnosed within 2 years of blood draw [0.36 (0.15–0.86)], though we did not find a significant risk reduction among AAs [0.75 (0.39–1.45)]. Notably, we found significant interactions between α-tocopherol and race after controlling the FDR to correct for multiple comparisons (Pinteraction = 0.02).
Conclusions: Our results indicate that plasma total-tocopherols are inversely associated with lung cancer risk, but the association may differ across specific isomeric forms of tocopherols, race, or other individuals’ characteristics. Further large-scale studies are warranted to confirm our findings.
Impact: Recommendations on tocopherols for lung cancer prevention should take isomers, race, and smoking behaviors into consideration.
This article was highlighted in the April issue.
Journal: Cancer Immunology Research
Neutrophils are the most abundant leukocytes in human blood and play a primary role in resistance against invading microorganisms and in the acute inflammatory response. However, their role in colitis and colitis-associated colorectal cancer is still under debate. This study aims to dissect the role of neutrophils in these pathologic contexts by using a rigorous genetic approach. Neutrophil-deficient mice (Csf3r−/− mice) were used in classic models of colitis and colitis-associated colorectal cancer and the role of neutrophils was assessed by histologic, cellular, and molecular analyses coupled with adoptive cell transfer. We also performed correlative analyses using human datasets. Csf3r−/− mice showed increased susceptibility to colitis and colitis-associated colorectal cancer compared with control Csf3r+/+ mice and adoptive transfer of neutrophils in Csf3r−/− mice reverted the phenotype. In colitis, Csf3r−/− mice showed increased bacterial invasion and a reduced number of healing ulcers in the colon, indicating a compromised regenerative capacity of epithelial cells. Neutrophils were essential for γδ T-cell polarization and IL22 production. In patients with ulcerative colitis, expression of CSF3R was positively correlated with IL22 and IL23 expression. Moreover, gene signatures associated with epithelial-cell development, proliferation, and antimicrobial response were enriched in CSF3Rhigh patients. Our data support a model where neutrophils mediate protection against intestinal inflammation and colitis-associated colorectal cancer by controlling the intestinal microbiota and driving the activation of an IL22-dependent tissue repair pathway.
This study was featured on the cover of the April issue.
Journal: Cancer Prevention Research
Serum miRNAs are promising biomarkers for several clinical conditions, including ovarian cancer. To inform equitable implementation of these tests, we investigated the effects of race, ethnicity, and socioeconomic status on serum miRNA profiles. Serum samples from a large institutional biobank were analyzed using a custom panel of 179 miRNA species highly expressed in human serum, measured using the Abcam Fireplex assay via flow cytometry. Data were log-transformed prior to analysis. Differences in miRNA by race and ethnicity were assessed using logistic regression. Pairwise t tests analyzed racial and ethnic differences among eight miRNAs previously associated with ovarian cancer risk. Pearson correlations determined the relationship between mean miRNA expression and the social deprivation index (SDI) for Massachusetts residents. Of 1,586 patients (76.9% white, non-Hispanic), compared with white, non-Hispanic patients, those from other racial and ethnic groups were younger (41.9 years ± 13.2 vs. 51.3 ± 15.1, P < 0.01) and had fewer comorbidities (3.5 comorbidities ± 2.7 vs. 4.6 ± 2.8, P < 0.01). On logistic regression, miRNAs predicted race and ethnicity at an AUC of 0.69 (95% confidence interval, 0.66–0.72), which remained consistent when stratified by most comorbidities. Among eight miRNAs previously associated with ovarian cancer risk, seven significantly varied by race and ethnicity (all P < 0.01). There were no significant differences in SDI for any of these eight miRNAs. miRNA expression is significantly influenced by race and ethnicity, which remained consistent after controlling for confounders. Understanding baseline differences in biomarker test characteristics prior to clinical implementation is essential to ensure instruments perform comparably across diverse populations.
Prevention Relevance: This study aimed to understand factors affecting miRNA expression, to ensure we create equitable screening tests for ovarian cancer that perform well in diverse populations. The goal is to ensure that we are detecting ovarian cancer cases earlier (secondary prevention) in women of all races, ethnic backgrounds, and socioeconomic means.
This study was featured on the cover of the April issue.

Journal: Cancer Research (April 1 Issue)
Eradication of acute myeloid leukemia (AML) is therapeutically challenging; many patients succumb to AML despite initially responding to conventional treatments. Here, we showed that the imipridone ONC213 elicits potent antileukemia activity in a subset of AML cell lines and primary patient samples, particularly in leukemia stem cells, while producing negligible toxicity in normal hematopoietic cells. ONC213 suppressed mitochondrial respiration and elevated α-ketoglutarate by suppressing α-ketoglutarate dehydrogenase (αKGDH) activity. Deletion of OGDH, which encodes αKGDH, suppressed AML fitness and impaired oxidative phosphorylation, highlighting the key role for αKGDH inhibition in ONC213-induced death. ONC213 treatment induced a unique mitochondrial stress response and suppressed de novo protein synthesis in AML cells. Additionally, ONC213 reduced the translation of MCL1, which contributed to ONC213-induced apoptosis. Importantly, a patient-derived xenograft from a relapsed AML patient was sensitive to ONC213 in vivo. Collectively, these findings support further development of ONC213 for treating AML.
Significance: In AML cells, ONC213 suppresses αKGDH, which induces a unique mitochondrial stress response, and reduces MCL1 to decrease oxidative phosphorylation and elicit potent antileukemia activity.
A commentary related to this study is available.
Journal: Cancer Research (April 15 Issue)
Fibroblasts in the Aged Pancreas Drive Pancreatic Cancer Progression
Pancreatic cancer is more prevalent in older individuals and often carries a poorer prognosis for them. The relationship between the microenvironment and pancreatic cancer is multifactorial, and age-related changes in nonmalignant cells in the tumor microenvironment may play a key role in promoting cancer aggressiveness. Because fibroblasts have profound impacts on pancreatic cancer progression, we investigated whether age-related changes in pancreatic fibroblasts influence cancer growth and metastasis. Proteomics analysis revealed that aged fibroblasts secrete different factors than young fibroblasts, including increased growth/differentiation factor 15 (GDF-15). Treating young mice with GDF-15 enhanced tumor growth, whereas aged GDF-15 knockout mice showed reduced tumor growth. GDF-15 activated AKT, rendering tumors sensitive to AKT inhibition in an aged but not young microenvironment. These data provide evidence for how aging alters pancreatic fibroblasts and promotes tumor progression, providing potential therapeutic targets and avenues for studying pancreatic cancer while accounting for the effects of aging.
Significance: Aged pancreatic fibroblasts secrete GDF-15 and activate AKT signaling to promote pancreatic cancer growth, highlighting the critical role of aging-mediated changes in the pancreatic cancer microenvironment in driving tumor progression.
A commentary related to this study is available.
Journal: Clinical Cancer Research (April 1 Issue)
Purpose: We explored the efficacy of PARP inhibition with or without programmed death ligand-1 (PD-L1) blockade as chemotherapy-free maintenance therapy for advanced triple-negative breast cancer (aTNBC) sensitive to platinum-based chemotherapy.
Patients and Methods: In the phase II non-comparative DORA trial (NCT03167619), patients with ongoing stable disease (SD) or complete/partial response (CR/PR) to first- or second-line platinum-based chemotherapy for TNBC (≤10% estrogen/progesterone receptor expression) were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1,500 mg on day 1 every 4 weeks. The primary objective was to compare progression-free survival (PFS) versus a historical control of continued platinum-based therapy.
Results: 45 patients were randomized (23 to olaparib alone, 22 to the combination; 3 with estrogen/progesterone receptor expression 1%–10%). At 9.8 months’ median follow-up, median PFS from randomization was 4.0 [95% confidence interval (CI), 2.6–6.1] months with olaparib and 6.1 (95% CI, 3.7–10.1) months with the combination, both significantly longer than the historical control (P = 0.0023 and P < 0.0001, respectively). Clinical benefit rates (SD ≥24 weeks or CR/PR) were 44% (95% CI, 23%–66%) and 36% (95% CI, 17%–59%) in the monotherapy and combination arms, respectively. Sustained clinical benefit was seen irrespective of germline BRCA mutation or PD-L1 status, but tended to be associated with CR/PR to prior platinum, particularly in the olaparib-alone arm. No new safety signals were reported.
Conclusions: PFS was longer than expected with both regimens. A patient subset with wild-type BRCA platinum-sensitive aTNBC had durable disease control with chemotherapy-free maintenance.
This article was highlighted in the April 1 issue.
Journal: Clinical Cancer Research (April 15 Issue)
Purpose: The study was to determine the activity and safety of the TGF-β inhibitor vactosertib in combination with imatinib in patients with desmoid tumors.
Patients and Methods: In this investigator-initiated, open-label, multicenter, phase Ib/II trial, patients with desmoid tumors not amenable to locoregional therapies (surgery and/or radiotherapy) or with disease progression following at least one treatment were enrolled. Participants were administered 400 mg imatinib daily in combination with vactosertib (5 days on and 2 days off, twice a day) every 28 days. In phase Ib, the vactosertib dose was set at 100 mg (level −1) and 200 mg (level 1) to determine the recommended phase II dose (RP2D). Phase II assessed the efficacy, with the primary endpoint being progression-free rate (PFR) at 16 weeks.
Results: No dose-limiting toxicities were observed during phase Ib; therefore RP2D was defined at doses of 400 mg imatinib daily in combination with 200 mg vactosertib. Of the 27 patients evaluated, 7 (25.9%) achieved a confirmed partial response and 19 (70.4%) were stable. The PFR at 16 weeks and 1 year were 96.3% and 81.0%, respectively. Most toxicities were mild to moderate myalgia (n = 10, 37%), anemia (n = 10, 37%), and nausea (n = 9, 33.3%). Common grade 3 to 4 toxicities included neutropenia (n = 6, 22.2%) and anemia (n = 5, 18.5%).
Conclusions: The vactosertib and imatinib combination was well tolerated, with promising clinical activity in patients with progressive, locally advanced desmoid tumors. This is the first study investigating a novel target agent, a TGF-β inhibitor, in this rare and difficult-to-treat desmoid tumor.
This study was highlighted in the April 15 issue.
Journal: Molecular Cancer Research
Identification of Colorectal Cancer Cell Stemness from Single-Cell RNA Sequencing
Cancer stem cells (CSC) play a critical role in metastasis, relapse, and therapy resistance in colorectal cancer. While characterization of the normal lineage of cell development in the intestine has led to the identification of many genes involved in the induction and maintenance of pluripotency, recent studies suggest significant heterogeneity in CSC populations. Moreover, while many canonical colorectal cancer CSC marker genes have been identified, the ability to use these classical markers to annotate stemness at the single-cell level is limited. In this study, we performed single-cell RNA sequencing on a cohort of 6 primary colon, 9 liver metastatic tumors, and 11 normal (nontumor) controls to identify colorectal CSCs at the single-cell level. Finding poor alignment of the 11 genes most used to identify colorectal CSC, we instead extracted a single-cell stemness signature (SCS_sig) that robustly identified “gold-standard” colorectal CSCs that expressed all marker genes. Using this SCS_sig to quantify stemness, we found that while normal epithelial cells show a bimodal distribution, indicating distinct stem and differentiated states, in tumor epithelial cells stemness is a continuum, suggesting greater plasticity in these cells. The SCS_sig score was quite variable between different tumors, reflective of the known transcriptomic heterogeneity of CRC. Notably, patients with higher SCS_sig scores had significantly shorter disease-free survival time after curative intent surgical resection, suggesting stemness is associated with relapse.
Implications: This study reveals significant heterogeneity of expression of genes commonly used to identify colorectal CSCs, and identifies a novel stemness signature to identify these cells from scRNA-seq data.
This article was highlighted in the April issue.
Journal: Molecular Cancer Therapeutics
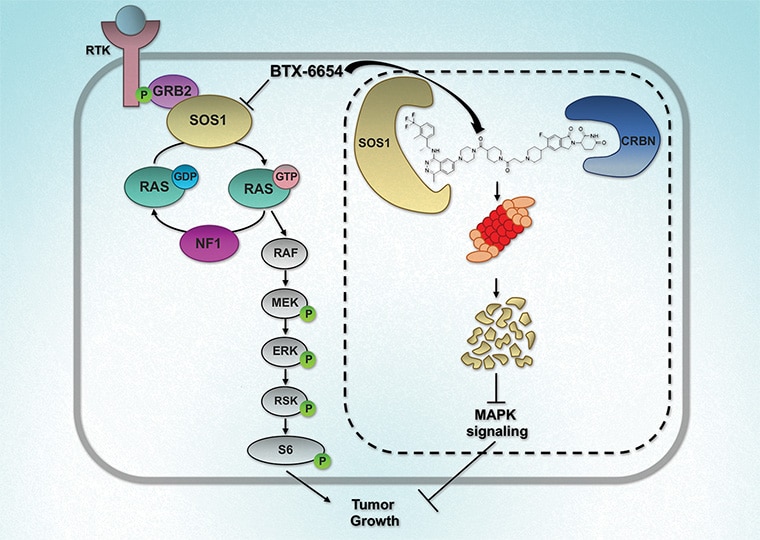
Mutations within the oncogene KRAS drive an estimated 25% of all cancers. Only allele-specific KRAS G12C inhibitors are currently available and are associated with the emergence of acquired resistance, partly due to upstream pathway reactivation. Given its upstream role in the activation of KRAS, son of sevenless homolog 1 (SOS1), has emerged as an attractive therapeutic target. Agents that target SOS1 for degradation could represent a potential pan-KRAS modality that may be capable of circumventing certain acquired resistance mechanisms. Here, we report the development of two SOS1 cereblon-based bifunctional degraders, BTX-6654 and BTX-7312, cereblon-based bifunctional SOS1 degraders. Both compounds exhibited potent target-dependent and -specific SOS1 degradation. BTX-6654 and BTX-7312 reduced downstream signaling markers, pERK and pS6, and displayed antiproliferative activity in cells harboring various KRAS mutations. In two KRAS G12C xenograft models, BTX-6654 degraded SOS1 in a dose-dependent manner correlating with tumor growth inhibition, additionally exhibiting synergy with KRAS and MEK inhibitors. Altogether, BTX-6654 provided preclinical proof of concept for single-agent and combination use of bifunctional SOS1 degraders in KRAS-driven cancers.
This study was highlighted and featured on the cover of the April issue.
Journal: Cancer Research Communications
Active surveillance (AS) is a suitable management option for newly diagnosed prostate cancer, which usually presents low to intermediate clinical risk. Patients enrolled in AS have their tumor monitored via longitudinal multiparametric MRI (mpMRI), PSA tests, and biopsies. Hence, treatment is prescribed when these tests identify progression to higher-risk prostate cancer. However, current AS protocols rely on detecting tumor progression through direct observation according to population-based monitoring strategies. This approach limits the design of patient-specific AS plans and may delay the detection of tumor progression. Here, we present a pilot study to address these issues by leveraging personalized computational predictions of prostate cancer growth. Our forecasts are obtained with a spatiotemporal biomechanistic model informed by patient-specific longitudinal mpMRI data (T2-weighted MRI and apparent diffusion coefficient maps from diffusion-weighted MRI). Our results show that our technology can represent and forecast the global tumor burden for individual patients, achieving concordance correlation coefficients from 0.93 to 0.99 across our cohort (n = 7). In addition, we identify a model-based biomarker of higher-risk prostate cancer: the mean proliferation activity of the tumor (P = 0.041). Using logistic regression, we construct a prostate cancer risk classifier based on this biomarker that achieves an area under the ROC curve of 0.83. We further show that coupling our tumor forecasts with this prostate cancer risk classifier enables the early identification of prostate cancer progression to higher-risk disease by more than 1 year. Thus, we posit that our predictive technology constitutes a promising clinical decision-making tool to design personalized AS plans for patients with prostate cancer.
Significance: Personalization of a biomechanistic model of prostate cancer with mpMRI data enables the prediction of tumor progression, thereby showing promise to guide clinical decision-making during AS for each individual patient.