Recognizing Cancer Immunotherapy Month
June 2022 marked the 10th Annual Cancer Immunotherapy Month spearheaded by the Cancer Research Institute.
Immunotherapy is an umbrella term describing a host of cancer treatments that activate different components of the immune system through distinct strategies but share the same basic idea: harnessing and boosting the body’s natural ability to recognize and fight cancer through immunity.
Almost 10 years after cancer immunotherapy was deemed “breakthrough of the year” by Science magazine in 2013 on the basis of encouraging results from several clinical trials, this form of cancer treatment has become standard of care for many different types of cancers and has extended the lives of countless patients.
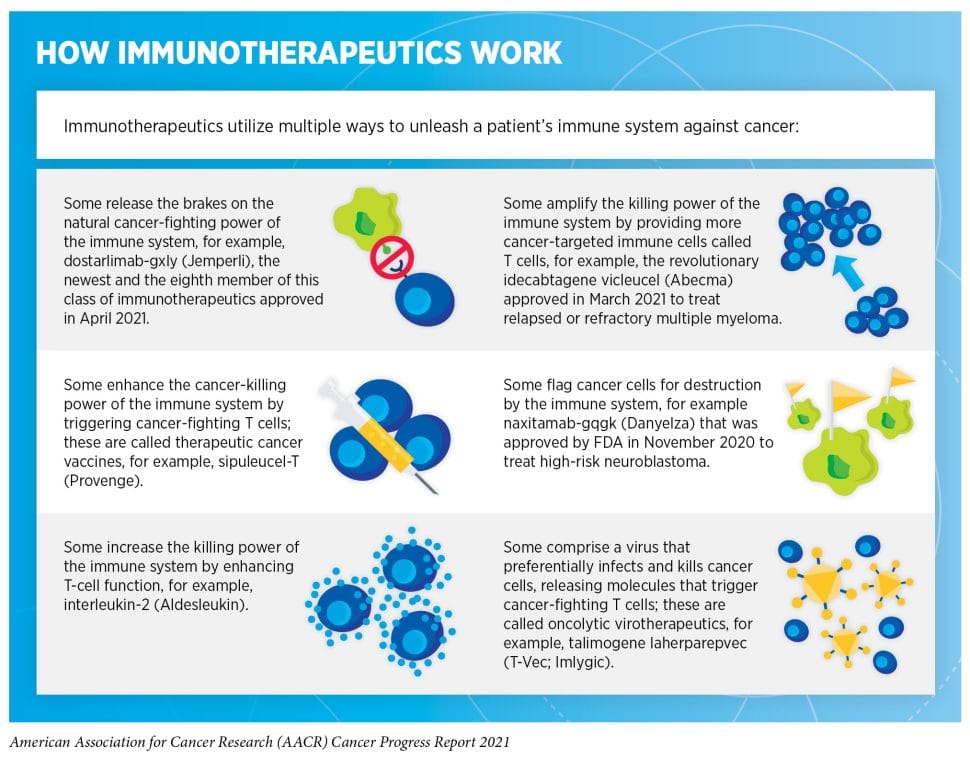
Although an antitumor cytokine, interferon alpha 2, was the first immunotherapy agent approved as a cancer treatment in 1986, the modern era of immunotherapy was ushered in by the discovery of immune checkpoint inhibitors that unleash the power of T cells against cancer cells. The first of these agents, ipilimumab (Yervoy), was approved in 2011 as a treatment for metastatic melanoma. Since then, several more immune checkpoint inhibitors have been approved, and the list of cancer indications has grown significantly.
Adoptive cell therapies, using a patient’s own immune cells engineered in the lab to boost their ability to find and kill cancer cells, have revolutionized the treatment of leukemia. The first chimeric antigen receptor (CAR) T-cell therapy was approved in 2017 for B-cell acute lymphoblastic leukemia (ALL). Recently, the first child to receive CAR T-cell therapy for ALL celebrated 10 years in remission.
Unfortunately, immunotherapy does not work for every cancer type, and not all patients who receive the treatment experience a response. In addition, many who initially benefit eventually develop therapy resistance. Some serious side effects related to the activation of the immune system are also a limitation.
Intense research is underway to enhance CAR T-cell therapy and leverage other types of immune cells, for example natural killer cells, to develop new cellular immunotherapies.
To improve efficacy, minimize treatment-associated toxicities, and overcome therapy resistance to immune checkpoint inhibitor therapy, researchers are investigating several approaches, including testing combination therapies, identifying biomarkers for patient selection, and treating earlier stages of the disease.
In a recent landmark study, a research team led by Luis A. Diaz Jr., MD, FAACR, reported success in treating a group of patients with locally advanced rectal cancer with immune checkpoint inhibitor therapy alone. All the patients involved in the study experienced complete remission and were spared the serious side effects of chemotherapy and radiation therapy. Diaz shared his thoughts on promising future directions in immunotherapy in a blog post earlier this year.
Also, check out our most recent blog posts covering progress in immunotherapy for brain and cervical cancer, and advances in single-cell analysis that are helping researchers assess the effects of immunotherapy on breast tumors.
For a deeper dive into some of the recent progress, see this collection of immunotherapy studies curated by the AACR journal Blood Cancer Discovery.