
FDA Approves New BTK-Targeted Therapeutic for Non-Hodgkin Lymphoma
Last week, the U.S. Food and Drug Administration (FDA) approved the molecularly targeted therapeutic acalabrutinib (Calquence) for treating adults...


Last week, the U.S. Food and Drug Administration (FDA) approved the molecularly targeted therapeutic acalabrutinib (Calquence) for treating adults...

This week’s excitement surrounding the groundbreaking U.S. Food and Drug Administration (FDA) approval of the CAR T–cell therapy tisagenlecleucel...

Earlier this week, the U.S. Food and Drug Administration (FDA) approved a new molecularly targeted therapeutic called neratinib (Nerlynx)...
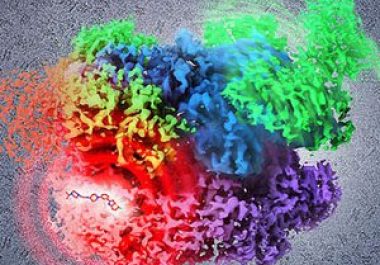
recent data suggest that there is another potential contender for a biomarker-based FDA approval: a targeted therapeutic called larotrectinib...

A study published recently in the AACR’s journal Cancer Discovery addresses the burgeoning question of the utility of high-throughput...

Last week, a flurry of U.S. Food and Drug Administration (FDA) oncology approvals concluded with the approval of the...

Earlier this week, the U.S. Food and Drug Administration approved the molecularly targeted therapeutic ribociclib (Kisqali) for use in...

Last week, the U.S. Food and Drug Administration (FDA) added a new therapeutic to the armamentarium for oncologists treating...

The U.S. Food and Drug Administration (FDA) recently approved the use of molecularly targeted therapeutic afatinib (Gilotrif) for treating...

Data presented at the AACR Annual Meeting 2016, on April 19, showed that a microdevice about the size of...